Monitoring for Per- and Poly-Fluoroalkyl (PFAS) with Advanced Mass Spectrometry– Based Methods
Per- and poly-fluoroalkyl substances (PFAS) are a family of potentially thousands of synthetic compounds that have long been used in the manufacture of a variety of common products with stain-repellent and nonstick properties. Their signature strong fluorine and carbon bonds make them difficult to break down and, as a result, they are among the most persistent of today’s environmental pollutants. Alarmingly, PFAS can be found in drinking water and have been shown to accumulate in the body with the potential to cause multiple health problems, such as hormone disruption and cancer. Advances in mass spectrometry have facilitated the detection of known PFAS contaminants as well as the identification of poorly studied and novel compounds in watersheds. This article explores the detection of known and novel PFAS contaminants in aqueous film-forming foams and raw drinking water sources in North Carolina, using new advances in mass spectrometry and data acquisition to improve identification and quantitation.
Per- and poly-fluoroalkyl substances (PFAS) hold the potential to cause multiple health problems in humans (1). One possible source of these pollutants is drinking water. Currently, there are no maximum contaminant levels (MCLs) established for PFAS chemicals in drinking water, but the United States Environmental Protection Agency (EPA) has taken steps to evaluate the need (2). It has also issued a health advisory for two key compounds, perfluorooctanoic acid (PFOA) and perfluorooctyl sulfonic acid (PFOS) (3), and in February 2019 published its PFAS action plan (4). In June of this year, the EPA officially established reporting thresholds for 172 PFAS and added these to the list of chemicals that must be reported to the Toxics Release Inventory, which tracks the management of toxic chemicals that may pose a threat to human health and the environment under the National Defense Authorization Act (5).
The nature of PFAS, and the large number of possible compounds that can be synthesized, mean that although many are known, a notable proportion of PFAS variants are being detected for which there are no existing analytical standards. There are more than 5000 viable PFAS variants (6), including precursors and telomers. PFAS have been manufactured and used extensively since the 1940s; they are found in many consumer products, including cookware, food containers, stain repellents, nonstick products, polishes, waxes, and cleaning materials. Manufacturing facilities using PFAS to make these and other products may contribute to their release into the environment. The aqueous film-forming foams (AFFF) used in firefighting at sites such as military bases and airports are considered to be a large source of PFAS groundwater contamination.
Having been in use for the longest time, PFOA and PFOS are the best documented PFAS in terms of chemical properties and toxicological effects. They are found to accumulate in the body, and the EPA has established health advisory levels at 70 ppt. Consequently, industries in the United States have phased out the production of PFOA and PFOS, but both persist in the environment and their manufacture continues in other parts of the world.
While much is known about managing the risks of PFOA and PFOS, insights are scarcer for newer PFAS such as chemicals from the Gen-X process used to make high-performance fluoropolymers without PFOA and perfluorobutane sulfonic acid (PFBS), a replacement for PFOS. In response to the growing understanding of PFAS, the EPA has issued draft toxicity assessments for these compounds (7).
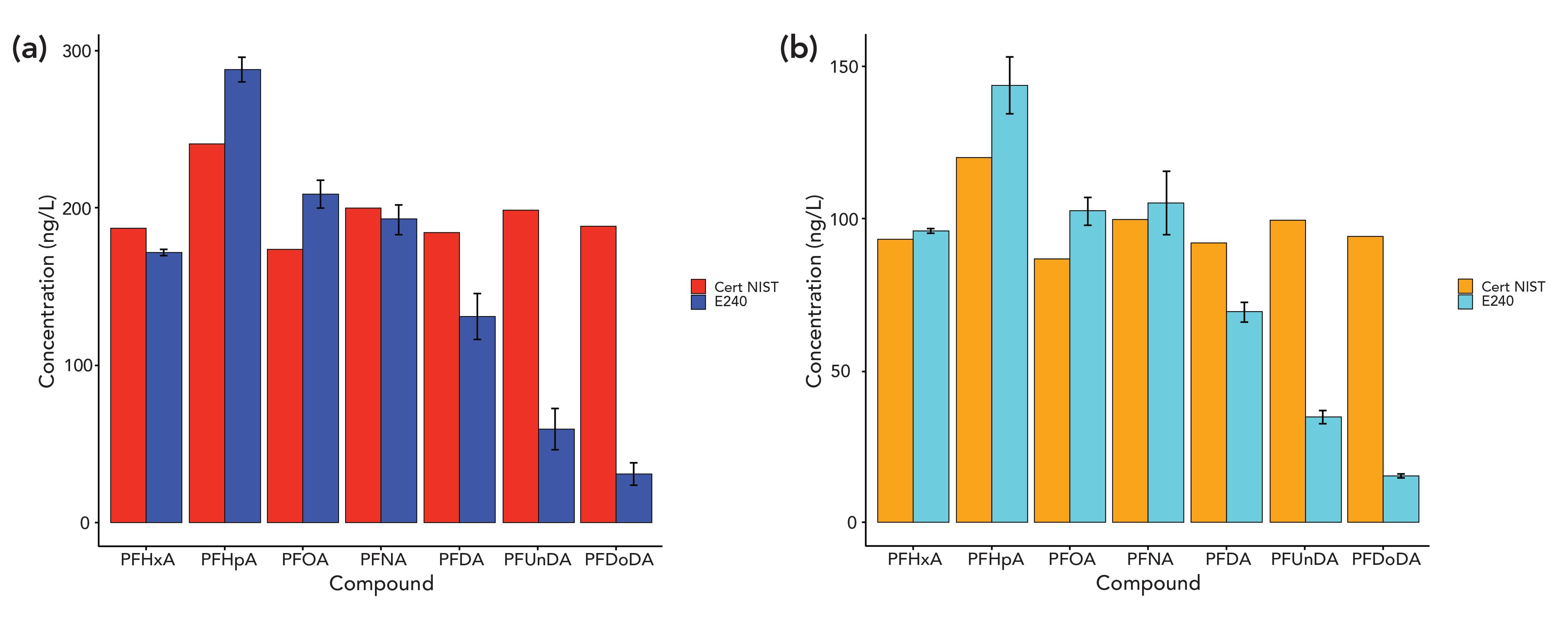
Figure 1: Quantitation performance for PFCAs in NIST SRM 8446. The red and orange bars are the certified NIST values, and the blue and teal bars are the measurements from this analysis. (a) is dilution 1 comparison, and (b) is dilution 2 comparison.
Environmental monitoring of PFAS is challenging because of the extensive range of novel compounds within this class that can be found in products or in the environment. In 2009, the EPA Office of Ground Water and Drinking Water developed a method specifically for the analysis of PFAS in drinking water based on solid-phase extraction (SPE), followed by liquid chromatography-tandem mass spectrometry (LC–MS/MS). In 2018, the method was updated and validated for additional PFAS compounds, including Gen-X (8). Given the health concerns surrounding this entire group of chemicals, the effective and reliable detection and quantitation of both known and novel compounds remains critical.
The sensitivity of LC–MS/MS instrumentation has continued to improve, and its usability for detecting polar compounds has made it the technique of choice for analyzing compounds of emerging concern such as PFAS in water. However, scientists undertaking targeted analysis of environmental contaminants have been limited to examining compounds for which analytical standards are available, and to the analytical methods necessary to identify those specific chemicals.
Advances in mass spectrometry now enable scientists to monitor established contaminants while detecting new analytes not on targeted screening lists. Recent developments in high-resolution, accurate-mass (HRAM)-MS offer high sensitivity along with a mass resolution that provides both accurate quantitation and screening capabilities for novel and nontargeted analytes. Advanced HRAM techniques and integrated data analysis strategies allow semi-quantitative analysis when standards are not available.
Both LC–MS/MS and HRAM have been used in work conducted by scientists at the Department of Civil and Environmental Engineering at Duke University to study PFAS in water. This group is part of the North Carolina Per and Polyfluoroalkyl Substances Testing (PFAST) Network, which is an academic consortium within the State of North Carolina and aims to assess the presence and distribution of PFAS compounds in the state’s public drinking water sources. The network takes both a targeted and nontargeted analytical approach to environmental analysis and testing, with nontargeted screening focused on identifying previously unknown and potentially toxic compounds.
North Carolina has many potential sources of PFAS contamination, including chemical manufacturing plants, textile production sites, military bases and fire departments. In recent years, compounds, such as Gen-X, have been detected in drinking water samples by PFAST network laboratories. In the work reported here, the goals were to: a) assess the sensitivity and quantitative performance of LC-HRAM for 48 targeted PFAS compounds in raw and finished drinking water matrices; b) perform non-targeted analysis of AFFF formulations for PFAS discovery; and c) analyze water extracts from a focused area of the Haw River in North Carolina for PFAS and emerging pollutants.
The Duke University laboratories implemented advanced analytical methods and instrumentation to achieve each of these three objectives.
Materials and Methods
The specifications of the Thermo Scientific Orbitrap Exploris 240 mass spectrometer used in this study are shown in Table I.
Targeted Analysis of Legacy and Emerging (GenX) PFAS
The analysis aimed to assess the sensitivity of the LC-HRAM for both legacy (PFOA) and emerging (GenX) PFAS, and verify its accuracy using a National Institute of Standards and Technology (NIST) standard reference material (SRM) for poly-fluorinated carboxylic acids (PFCAs). Direct injection (50 μL) of raw and finished drinking water matrices was performed by using the LC–HRAM-MS instrument, targeting 48 PFAS compounds. The process required minimal sample preparation with samples centrifuged at 11,000 x g (times gravity) for 5 min to remove particles, and the addition of 2% methanol containing 20 stable isotope-labeled PFAS surrogates at a final concentration of 50 ng/L per compound. Full scan mode was used for quantitation, with no SPE step. Two separate ion transfer tube temperatures were used for emerging (such as ether acid) and legacy PFAS compounds, due to many emerging PFAS’ susceptibility to thermal degradation, and performed two separate injections: The first at 150 °C for ether acids and at 325 °C for legacy PFAS compounds. Differing electrospray ionization (ESI) spray voltages (3500 V vs. 1500 V) and sheath gas flows (40 vs. 30 units) were also used for legacy compounds and ether acids, respectively. The full scan was performed at a resolution setting of 120,000.
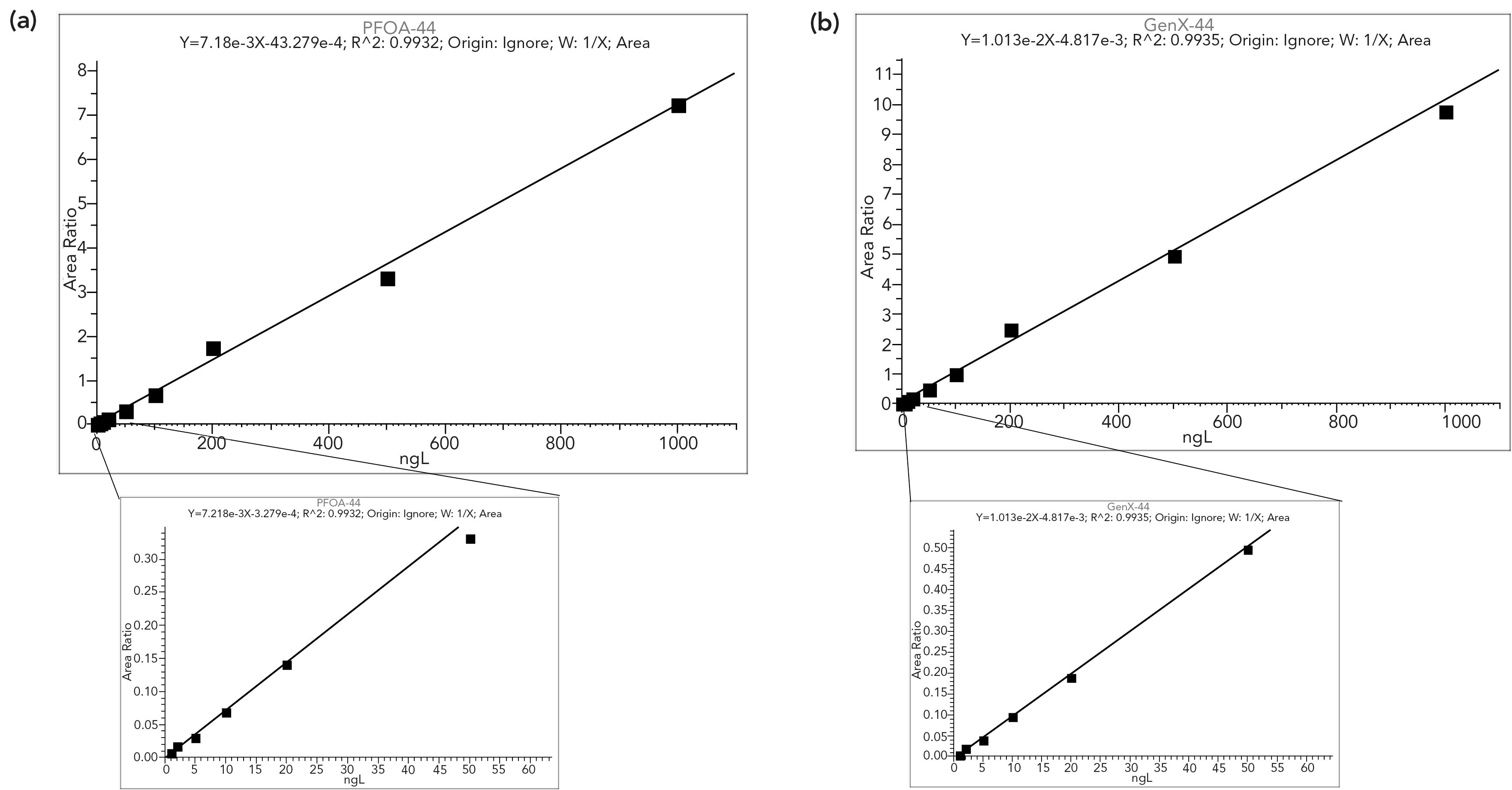
Figure 2: The results showed excellent sensitivity for legacy (PFOA) and emerging (GenX) PFAS, (a) is PFOA-44, and (b) is GenX-44.
Nontargeted Analysis of PFAS in AFFF
For analysis of suspected PFAS in AFFF, analysis was conducted by the Thermo Scientific AcquireX data acquisition workflow to maximize MS/MS coverage for potentially low-abundance but interesting PFAS compounds. This approach was applied to nontargeted discovery analysis of PFAS in six AFFF formulations. These formulations were first diluted 10,000 times, followed by five sequential deep-scan injections for data-dependent acquisition (DDA) with both positive and negative ESI polarities.
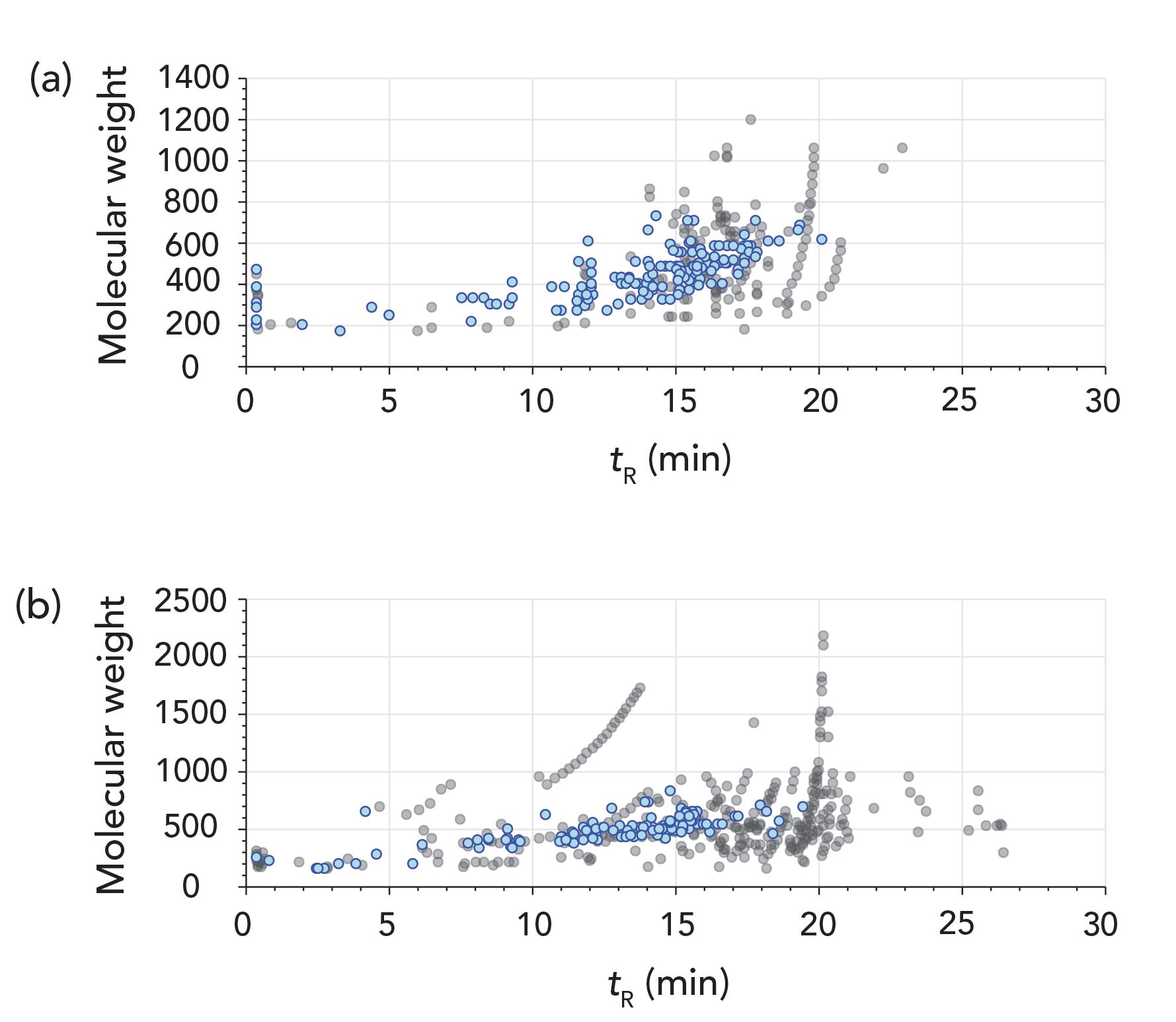
Figure 3: Nontargeted analysis of PFAS in aqueous film-forming foams: Putative PFAS compounds are detected in ESI(±). Blue dots are putative PFS (140 negative, 116 positive) and black dots mostly hydrocarbons (common alkyl ethoxylates). (a) is ESI(-) and (b) is ESI(+).
The workflow enabled a sequential inclusion–exclusion approach, in which matrix, system, and background precursor features deemed irrelevant were mapped, imported into the DDA method, and excluded from MS data acquisition of the samples of interest. This list was then fed into the first sample analysis (full- scan MS detection) to create an “inclusion list” composed of compounds of interest. Subsequently, an iterative DDA process (with both exclusion and inclusion lists) was performed, with multiple injections after each run, and the lists were refined and updated throughout according to the MS/MS results.
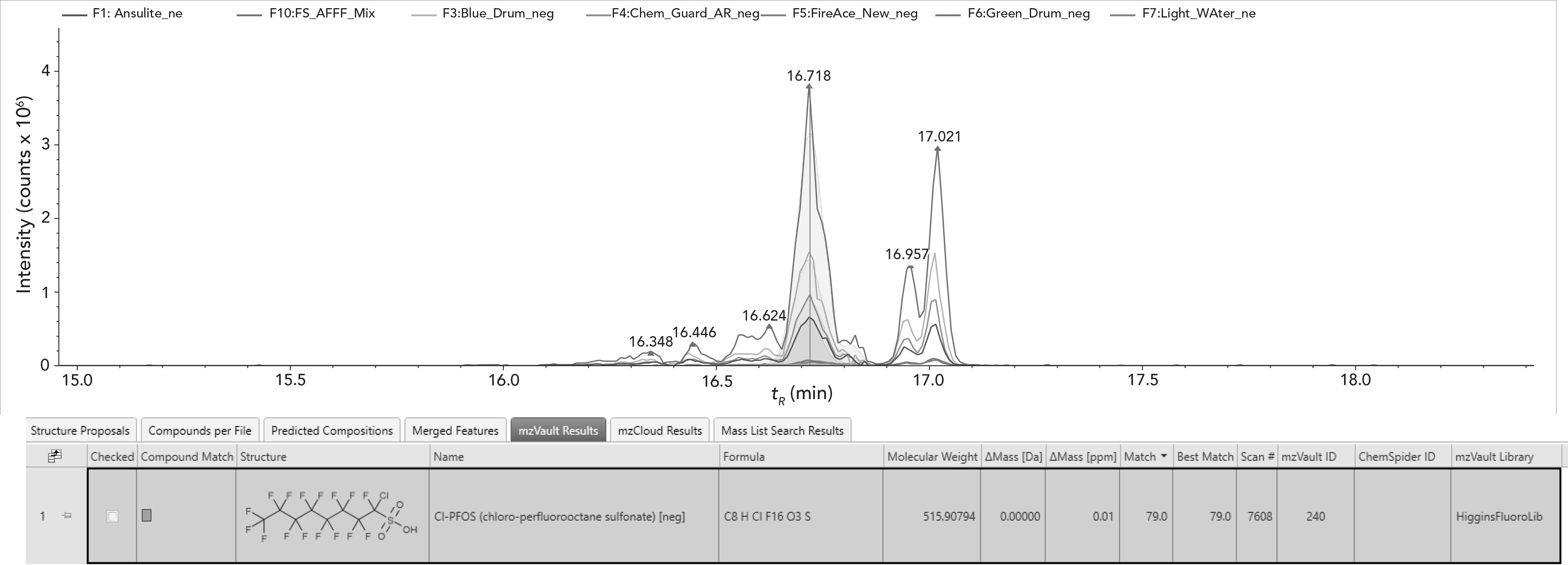
Figure 4: Novel PFAS annotation via custom mzVault library.
Targeted and Nontargeted Analysis of PFAS in North Carolina’s Haw River
The workflow was used to analyze 13 water samples via three sequential DDA MS/MS injections of two composite water mixtures, to assess levels of PFAS and other emerging contaminants. The two mixtures were created—one combining six of the 13 samples and one combining seven—and subjected to a full DDA MS/MS deep-scan workflow. Both direct injection and SPE were used in sample preparation. Data was subsequently analyzed using the Compound Discoverer 3.1 software (Thermo Scientific) to annotate nontargeted compounds with molecular and structural formulae, where possible.
Results
We first assessed the quantitative accuracy of our MS approach by testing for PFCAs in NIST SRM 8446 (which contains certified levels of PFCAs). The analysis saw generally good agreement in two different dilutions for PFCAs with perfluoroalkyl chains up to C10 (such as poly-fluorinated decanoic acid; see Figure 1), although compounds with higher molecular weights may have been lost due to the specifics of the highly aqueous solution used in the analysis (which may have caused adsorption onto vials or sample collection containers).
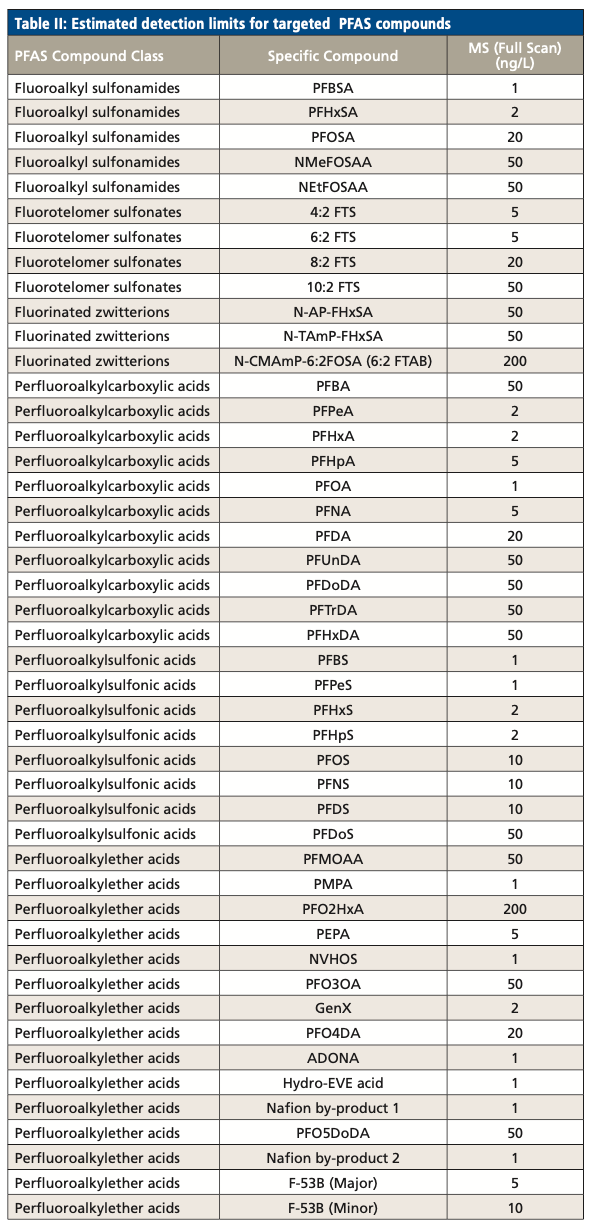
The analysis showed excellent sensitivity for targeted compounds, specifically GenX. For some, especially intermediate alky-chain length, compounds, we achieved detection limit sensitivities of between 1 and 5 ng/L; for some longer-chain compounds and also PFBA, sensitivities ranged up to 50 ng/L. For GenX, the estimated detection limit was 2 ng/L and overall detection limits achieved good sensitivity in the range of low-to-mid parts per trillion (as shown in Table II). Overall, calibration curves for legacy PFAS (PFOA) and emerging PFAS (GenX) were linear over a wide concentration range, as shown in Figure 2.
For nontargeted analysis of PFAS in AFFF, 307 total compounds were detected from six AFFF formulations in ESI(-) and 449 in ESI(+). The DDA analysis achieved 100% DDA MS/MS acquisition coverage for both polarities (over all five injections). From these compounds, 140 putative PFAS compounds were annotated in ESI(-) and 116 PFAS in ESI(+), which is seen in Figure 3. However, some redundant detections occurred due to branched isomers (where substances are similar in structure, but different in retention time).
In the Haw River water samples, the analysis detected 6767 total com- pounds in 13 water samples in ESI(-), with >90% DDA MS/MS acquisition coverage over three sequential injections. Using a harmonized set of spectral libraries (Thermo Scientific mzCloud mass spectral library, NIST MS/MS, and MassBank of North America), 228 “unique” library matches were obtained with MS/MS match scores greater than 80%. These were further analyzed using a hierarchical cluster approach to match samples to sites and were also used to check instrument mass accuracy with no mass accuracy errors exceeding 0.5 ppm. Such high mass accuracy is needed to perform trace level nontargeted screening work with confidence.
Additionally, using our LC–HRAM-MS/MS workflow and subsequent spectral analysis methodology, we were able to annotate some novel PFAS compounds in AFFF very easily using the Compound Discoverer software with spectra contained in the mzVault library. An example of a compound detected with a formula of C8HClF16O3S is shown in Figure 4 with annotation and library spectral matching as a mirror plot on the right.
Due to its inclusion-exclusion functionality, the DDA methodology with the workflow eliminated the need for data-independent acquisition (DIA) to ensure MS/MS coverage. As well as utilizing this approach for emerging and legacy PFAS analysis, we tested it on EPA’s non-targeted analysis collaborative trial (ENTACT) samples. The DDA strategy (three sequential injections, updated exclusion/inclusion lists) resulted in 85–100% MS2 coverage, compared to <60% with standard DDA (three replicate injections, intensity-dependent precursor selection). This demonstrates a high-coverage method without reliance on DIA strategies.
Conclusion
In the studies reported here for analysis of waters in North Carolina, highly sensitive targeted PFAS analysis by direct-injection LC–MS/MS was used to understand the occurrence of PFAS in drinking water sources, and an intelligent data acquisition strategy was employed to mediate the characterization of PFAS found in water samples from the Haw River and six AFFF formulations commonly used at firefighting sites.
The techniques enabled both quantitative and semiquantitative, targeted and nontargeted analysis of legacy and emerging PFAS com- pounds. The workflow provides high sensitivity for quantitative analysis of PFAS in drinking water sources through direct-injection LC–HRAM-MS, and the combination of MS and the DDA strategy offers more complete MS/MS coverage for analysis of PFAS in firefighting foam. The workflow’s excellent mass accuracy (<0.5 ppm achieved in practice), coupled with expanded HRAM-MS/MS spectral libraries, allows high-confidence compound annotation in nontargeted analysis of water samples, which in turn al- lows the accurate identification and characterization of novel, and potentially harmful, PFAS in drinking water sources.
References
(1) United States Environmental Protection Agency. PFOA, PFOS and Other PFAS. https://www.epa.gov/ pfas/basic-information-pfas
(2) United States Environmental Protection Agency. EPA’s Third Un- regulated Contaminant Monitoring Rule: https://www.epa.gov/dwucmr/third-unregulated-contaminant-monitoring-rule
(3) United States Environmental Protection Agency. EPA’s Drinking Water Health Advisories for PFOA and PFOS. https://www.epa.gov/ ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos
(4) United States Environmental Protection Agency. EPA’s PFAS Action Plan. https://www.epa.gov/pfas/epas-pfas-action-plan
(5) United States Environmental Protection Agency. Addition of Certain PFAS to the TRI by the National Defense Authorization Act. https://www.epa. gov/toxics-release-inventory-tri-program/addition-certain-pfas-tri-national-defense-authorization-act
(6) United States Environmental Protection Agency. EPA’s PFAS Master List of PFAS Substances. https://comptox. epa.gov/dashboard/chemical_lists/ PFASMASTER
(7) United States Environmental Protection Agency. EPA’s Fact Sheet: Draft Toxicity Assessments for GenX Chemicals and PFBS. https://www.epa. gov/sites/production/files/2018-11/ documents/factsheet_pfbs-genx-tox- icity_values_11.14.2018.pdf
(8) United States Environmental Protection Agency. Method 537.1: Determination of selected per- and polyfluorinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/ tandem mass spectrometry (LC/ MS/MS). https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=343042&Lab=NERL
P. Lee Ferguson is an Associate Professor of Civil and Environmental Engineering at Duke University in Durham, North Carolina. Maciej Bromirski is a Product Manager at Thermo Fisher Scientific in Wroclaw, Poland, and Siegrun Mohring is a Product Specialist at Thermo Fisher Scientific in Bremen, Germany. Direct correspondence to: maciej.bromirski@thermofisher.com
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.









