High-Throughput Profiling of Long Chain Fatty Acids and Oxylipins by LC–MS
Long chain fatty acids (LCFAs) function as a source of metabolic energy, substrates for membrane biogenesis, and storage of metabolic energy. Oxylipins, oxygenated derivatives of LCFAs, regulate the activity of many cellular processes. Existing methods for the analysis of LCFAs and oxylipins have limited compound coverage and sensitivity that, therefore, prevent their application in biological studies. In this work, we developed a high-throughput LC–MS method for analysis of 51 LCFAs and oxylipins. LCFAs and oxylipins were first extracted from biological samples via solid-phase extraction. The extracted molecules were analyzed by targeted comparative metabolomics. Saturated and monounsaturated LCFAs were analyzed in single ion reaction mode, while polyunsaturated LCFAs and oxylipins were analyzed in multiple reaction monitoring mode. Using this method, we successfully quantified 31 LCFAs and oxylipins from mouse livers.
Long chain fatty acids (LCFAs) are fatty acids with aliphatic tails of 13 to 21 carbons and are classified as saturated, monounsaturated, or polyunsaturated LCFAs, depending on the number of double bonds. LCFAs function as a source of metabolic energy, substrates for biogenesis of membrane phospholipids, and storage of metabolic energy through triglycerides and cholesterol ester. Oxylipins, oxygenated derivatives of polyunsaturated fatty acids (PUFAs), are derived from PUFAs via cyclooxygenase (COX), lipoxygenase (LOX), cytochrome P450 (CYP450), and nonenzymatic oxidation pathways (1,2). Oxylipins regulate, directly or indirectly, the activity of various biological processes, including apoptosis, tissue repair, blood clotting, cell proliferation, blood vessel permeability, pain sensation and perception, inflammation, immune actions, and blood pressure regulation (3–5).
Different analytical methods, such as radiometric and enzymatic immunoassays, have been developed to analyze LCFAs and oxylipins (6–8). However, these approaches cover only a limited number of compounds and often lack specificity. Gas chromatography-mass spectrometry (GC–MS) has been employed to detect a wide range of oxylipins (9). Although GC–MS is sensitive, compounds need to be derivatized to increase their thermal stability and volatility. Derivatization not only requires laborious and time-consuming sample preparation but also introduces variation in compound quantification and identification.
The objective of this study was to develop a liquid chromatography–mass spectrometry (LC–MS) method to simultaneously quantify a large number of LCFAs and oxylipins via targeted comparative metabolomics (10). We extracted LCFAs and oxylipins from biological samples via solid-phase extraction (SPE). The extracted compounds were then analyzed by ultra-high-performance liquid chromatography electrospray ionization-tandem mass spectrometry (UHPLC-ESI-MS/MS) via targeted comparative metabolomics for the profiling of 51 bioactive LCFAs and oxylipins. The developed method was applied to quantify LCFAs and oxylipins in mouse liver. Considering the biological roles of LCFAs and oxylipins, the developed method could become an essential method in nutritional and clinical research.
Experimental
Chemicals and Solvents
LCFA and oxylipin standards were purchased from Cayman Chemicals and Sigma-Aldrich. Acetonitrile and acetic acid are HPLC grade or higher and were purchased from Fisher Scientific.
Animal Treatment and Sample Collection
Eight-week-old male (C57BL/6J) mice were purchased from the Jackson Laboratory. Mice were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Food and tap water were allowed ad libitum. The procedures of animal care were approved by the University of Louisville Institutional Animal Care and Use Committee. Two groups of C57BL/6J mice were fed different diets. One group was fed an isocaloric maltose dextrin solution (Group 1, n = 5) while the other group was fed with a Lieber DeCarli liquid diet containing 5% alcohol (Group 2, n = 8). After a 10-day dietary intervention, mice were anesthetized with ketamine:xylazine (100mg:15 kg i.m.). The liver was collected from each mouse, frozen in liquid nitrogen, and stored in a 1.5 mL Eppendorf tube at −80 °C until use.
Sample Preparation
Liver samples were homogenized using 50% ethanol with 0.1% butylated hydroxytoluene (BHT) at the ratio of 1 mg tissue per 20 μL solvent. SPE was performed to extract LCFAs and oxylipins from liver tis- sue using a Waters Oasis HLB cartridge (1 mL, 30 mg, 30 μm). The cartridge was conditioned with 1 mL methanol followed by equilibration with 1 mL water. After loading 400 μL homogenized liver sample, the cartridge was washed with 1 mL 5% methanol (v/v). LCFAs and oxylipins were eluted with 1 mL methanol/acetonitrile (10/90, v/v). The extract was evaporated to dryness under a stream of nitrogen and then redissolved in 100 μL 50% ethanol for LC–MS analysis.
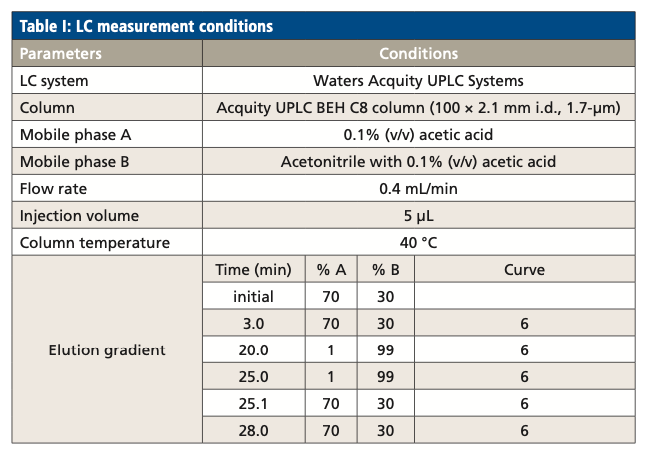
LC Measurement Conditions
LCFAs and oxylipins were separated by reversed-phase chromatography. The parameters for the LC separation are listed in Table I.
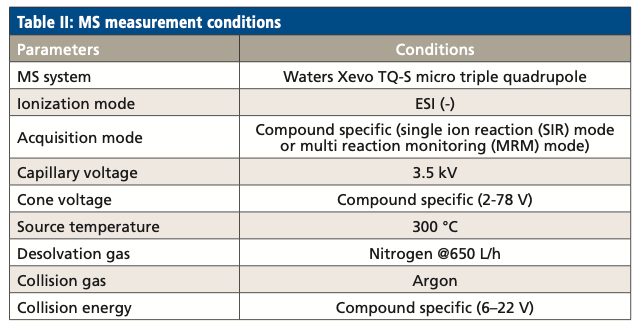
MS Measurement Conditions
LCFAs and oxylipins eluted from the LC system were analyzed by a triple-quadrupole mass spectrometer. The mass spectrometry parameters are listed in Table II.
Results and Discussion
Optimization of LC–MS
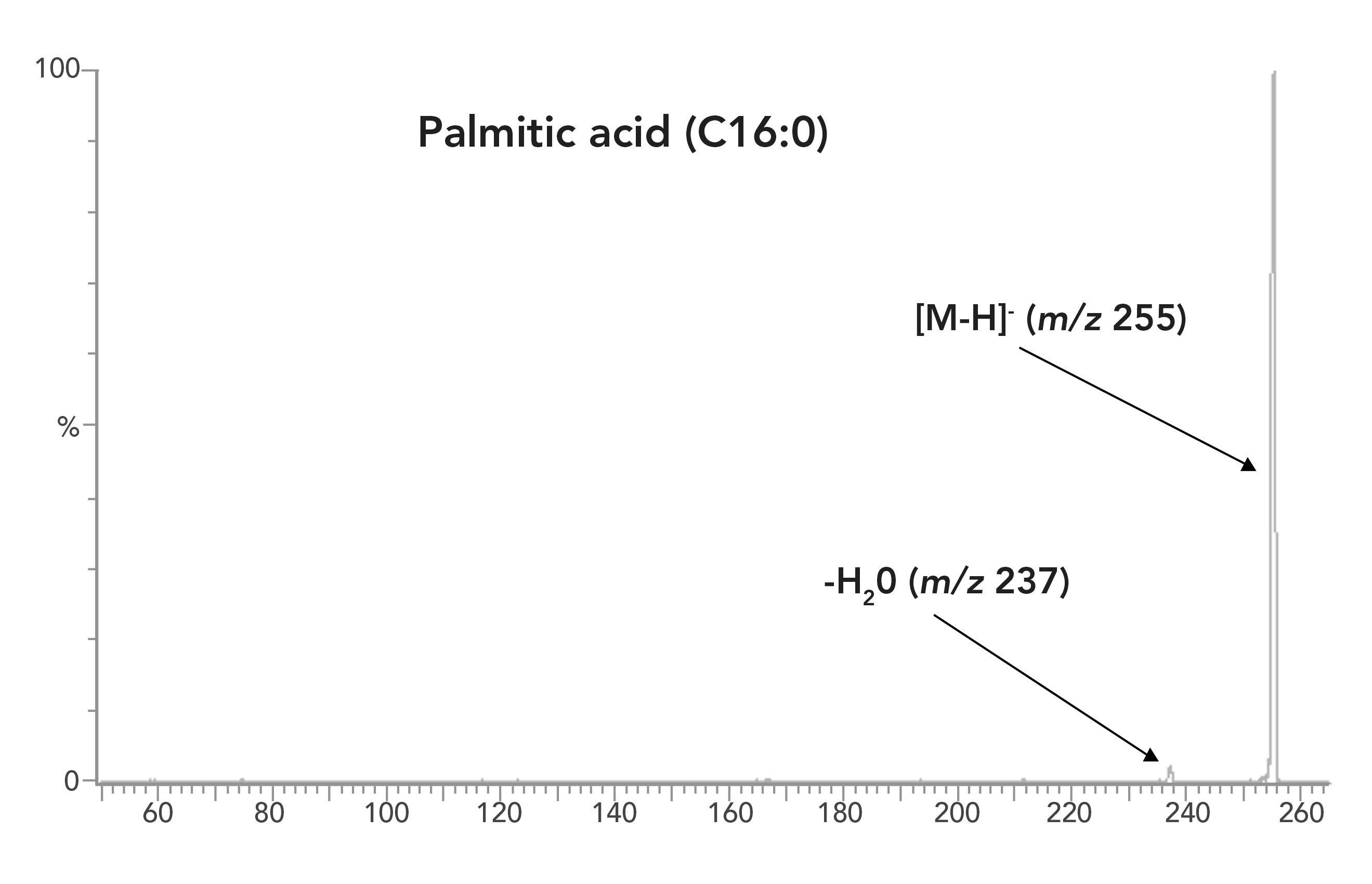
All LCFAs and oxylipins were initially analyzed in multiple reaction modeling (MRM) mode to minimize interferences from the matrix. However, the fragmentation efficiency of saturated and monounsaturated LCFAs was too low to achieve satisfactory sensitivity. For example, the most abundant daughter ion of palmitic acid (C16:0) is [M-18]-, an ion formed by the loss of H2O from the carboxylic group. The abundance of this ion is less than 5% of the parent ion (Figure 1). Analysis of the saturated and monounsaturated LCFAs in selective ion recording mode provides a better sensitivity, even though more analytical interferences occur in the SIR mode than in the MRM mode.
Figure 1: Fragmentation of palmitic acid (C16:0) in MRM mode.
The selection of optimal MRM transitions was achieved by direct infusion of a solution of the individual compound standard at a flow rate of 15 μL/min. The intensity of parent ion was optimized by varying the cone voltage while the abundant daughter ions were observed by varying the collision energy. Up to five transitions were initially generated for each compound, from which the most intense or selective transition was manually determined.
The LC separation took 28 min, including 6 min of column equilibration. This running time is comparable with methods reported in the literature for LC analysis of eicosanoids (11,12). The retention time of an LCFA depends on the length of the fatty acyl chain and the number of double bonds. As shown in Figure 2, using reversed-phase liquid chromatography (RPLC) separation, LCFAs with a shorter fatty acyl chain or a large number of double bonds eluted earlier than those with longer fatty acyl chain or a smaller number of double bonds. For example, arachidic acid (C20:0) eluted at 17.95 min, stearic acid with a shorter fatty acyl chain (C18:0) was eluted at 16.62 min, and EPA with more double bonds (C22:5) was eluted at 13.70 min.
Figure 2: Chromatograms of LCFAs. The left panel shows LCFAs with the same number of double bonds but different carbon numbers. The right panel shows LCFAs with the same carbon number but varying numbers of double bonds. Note for chromatograms that the x-axis is time (min) and the y-axis is signal intensity.
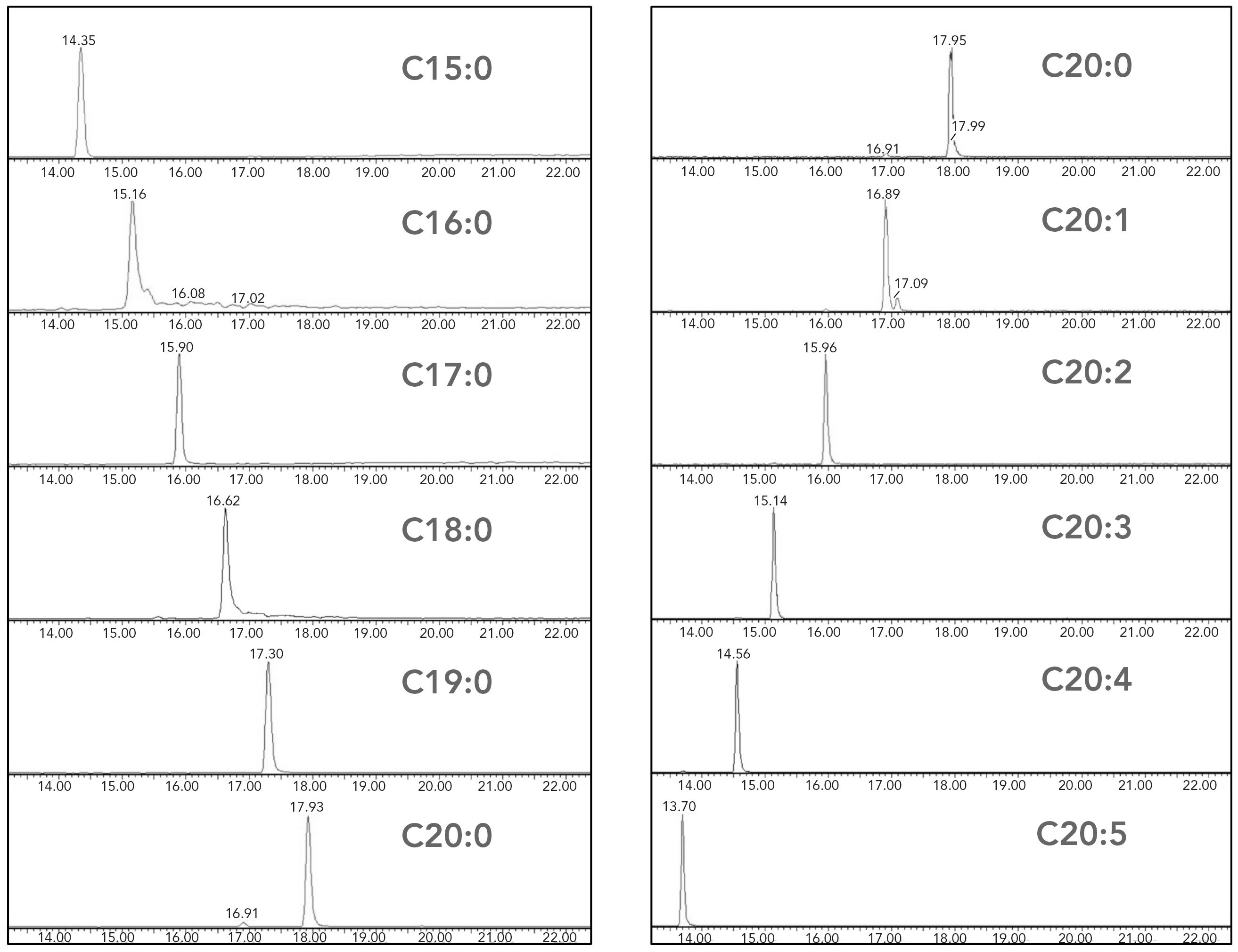
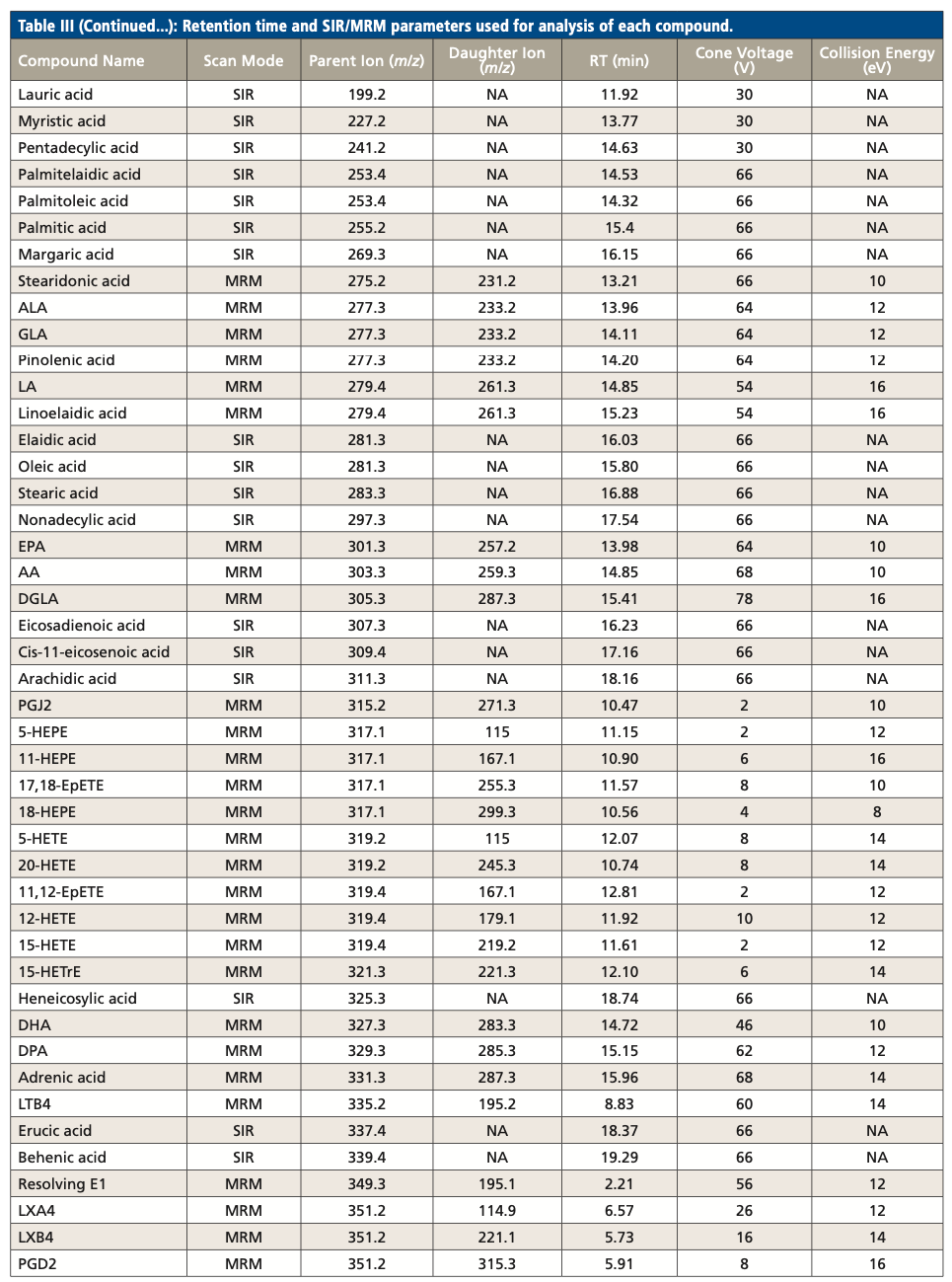
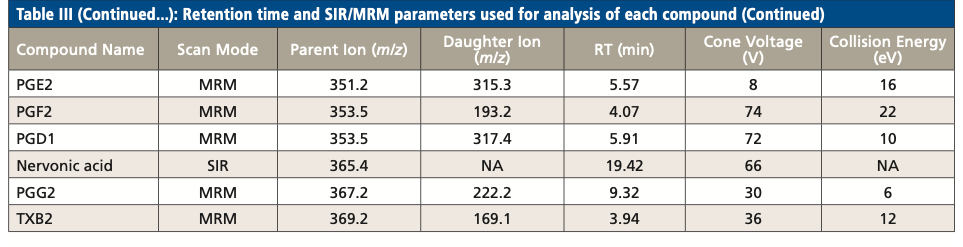
Table III summarizes the retention times and parameters used in SIR and MRM for the analysis of each LCFA and oxylipin, including parent ion m/z, cone voltage, daughter ion m/z, and collision energy. While several compounds have the same MRM transition, they were separated by RPLC. For example, PGD2 and PGE2 have the same MRM transition of 351.2 > 315.3. They were eluted at 5.91 min and 5.57 min respectively. On the other hand, several coeluted compounds were differentiated by their unique MRM transition. For example, both PGD1 and PGD2 eluted at 5.91 min, but their MRM transitions were 353.5 > 317.4 and 351.2 > 315.3, respectively.
Quantification of LCFAs and Oxylipins in Biological Samples
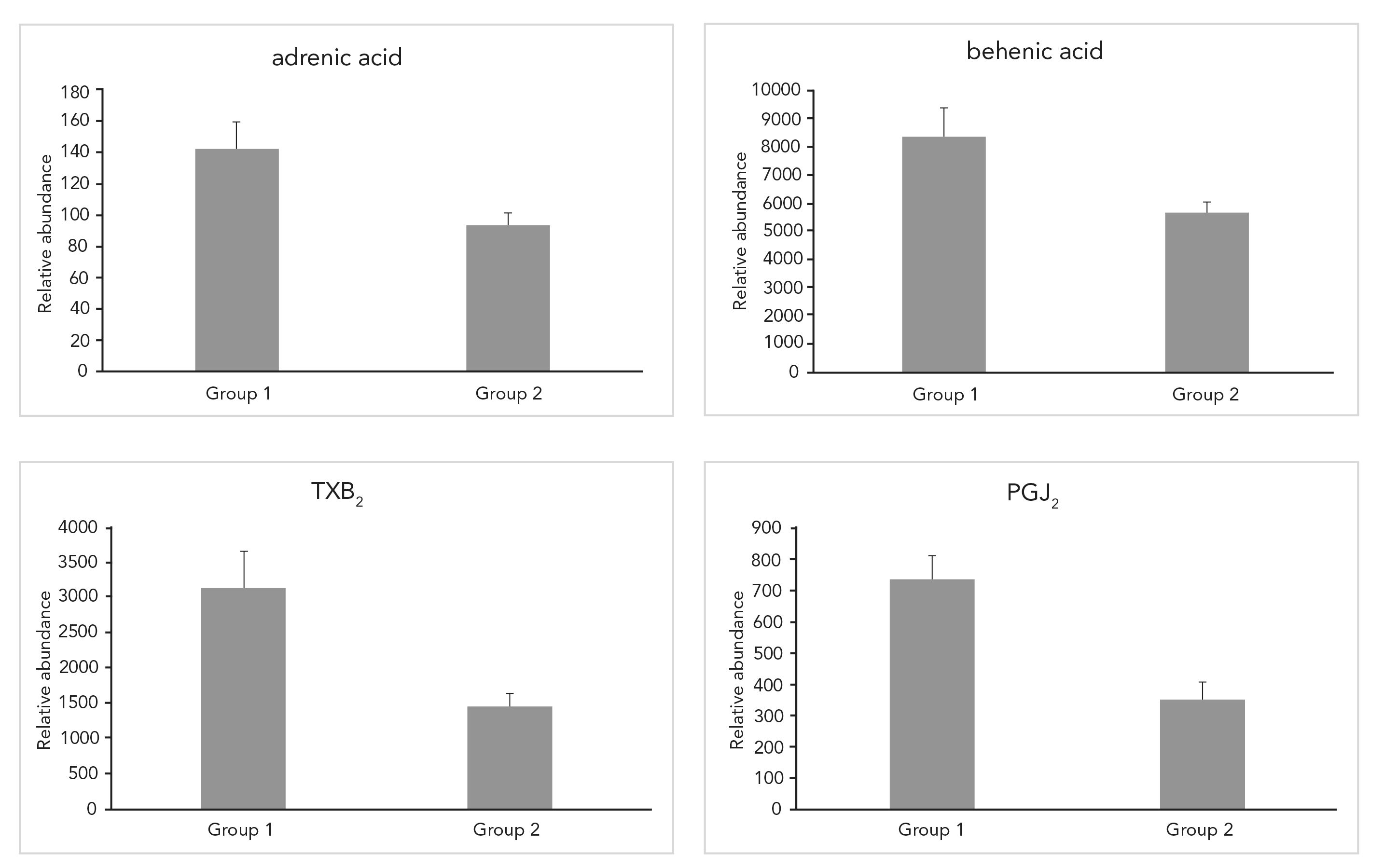
We extracted LCFAs and oxylipins from mouse livers and analyzed the extracted compounds using the developed method. A total of 31 LCFAs and oxylipins were detected in mouse livers. Their relative abundances are listed in Table IV. We performed a pairwise two-tail t-test to study the abundance difference of the detected 31 compounds between the two groups, Group 1 and Group 2. A compound was considered to have a significant abundance difference between two groups if its p-value of the t-test is less than 0.05. Four compounds (adrenic acid, behenic acid, TXB2, and PGJ2) were down-regulated after the animals were fed alcohol (Figure 3). A detailed discussion of the biological importance of these results will be disclosed in another report.
Figure 3: LCFAs and oxylipins with significant differences in abundance after alcohol-fed Group 1 are pare-fed mice, and Group 2 are alcohol-fed mice for (a) adrenic acid, (b), behenic acid, (c) TXB2, and (d) PGJ2.
Conclusion
We developed a high throughput LC–MS method for the simultaneous analysis of 51 bioactive LCFAs and oxylipins. Polyunsaturated LCFAs and oxylipins were analyzed in MRM mode. Saturated and monounsaturated LCFAs were analyzed in SIR mode, owing to the better sensitivity of SIR mode for these compounds, even though more analytical interference was introduced. Using the developed method, we successfully quantified 31 LCFAs and oxylipins in mouse livers and detected four compounds with significant abundance alteration after alcohol treatment. Considering the biological roles played by LCFAs and oxylipins, this method could become an essential method in nutritional and clinical research, biomarker development, as well as drug discovery and development.
Acknowledgments
The authors thank Mrs. Marion McClain for review of this manuscript. This work was supported by NIH [S10OD020106 (XZ); 1P20GM113226 (CJM); 1P50AA024337 (CJM); 1U01AA026934 (CJM); 1U01AA026936 (CJM); 1U01AA026980 (CJM); and 1R01AA023681 (CJM)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Department of Veterans Affairs, 1I01BX002996-01A2 (CJM).
References
(1) C. Westphal, A. Konkel, and W.H. Schunck, Cytochrome P450 Enzymes in the Bioactivation of Polyunsaturated Fatty Acids and Their Role in Cardiovascular Disease, E.G. Hrycay, and S.M. Bandiera, Eds. (Springer International Publishing, Cham, Switzerland, 2015), pp. 151–187.
(2) C. Vigor, J. Bertrand-Michel, E. Pinot, C. Oger, J. Vercauteren, P. Le Faouder, J.M. Galano, J.C.Y. Lee, and T. Durand, J. Chromatogr. B. 964, 65–78 (2014).
(3) M.W. Buczynski, D.S. Dumlao, and E.A. Dennis, J. Lipid Res. 50, 1015–1038 (2009).
(4) C. Arnold, A. Konkel, R. Fischer, and W.H. Schunck, Pharmacol. Rep. 62, 536–547 (2010).
(5) M. Gabbs, S. Leng, J.G. Devassy, M. Monirujjaman, and H.M. Aukema, Adv. Nutr. 6(5), 513–540 (2015).
(6) J.H. Dahl and R.B. van Breemen, Anal. Biochem. 404(2), 211–216 (2010).
(7) F. Dray, B. Charbonnel, and J. Maclouf, Eur. J. Clin. Invest. 5(4), 311– 318 (1975).
(8) B.M. Jaffe, H.R. Behrman, and C.W. Parker, J. Clin. Invest. 52(2), 398–405 (1973).
(9) D. Tsikas, J. Chromatogr. B Biomed. Appl. 717, 201–245 (1998).
(10) C. Gonzalez-Riano, D. Dudzik, A. Garcia, A. Gil-de-la-Fuente, A. Gradillas, J. Godzien, A. Lopez-Gonzalvez, F. Rey-Stolle, D. Rojo, F.J. Ruperez, J. Saiz, and C. Barbas, Anal. Chem. 92, 203–226 (2020).
(11) A. Margalit, K.L. Duffin, and P.C. Isakson, Anal. Biochem. 235(1), 73–81 (1996).
(12) K. Nithipatikom, R.F. DiCamelli, S. Kohler, R.J. Gumina, J.R. Falck, W.B. Campbell, and G.J. Gross, Anal. Biochem. 292, 115–124 (2001).
Xiang Zhang, Fang Yuan, and Liqing He are with the Department of Chemistry in the Center for Regulatory and Environmental Analytical Metabolomics at the University of Louisville Alcohol Research Center, in Louisville, Kentucky. Wenke Feng and Craig J. McClain are with the Departments of Pharmacology & Toxicology and Medicine at the University of Louisville, in Louisville, Kentucky. Direct correspondence to: xiang.zhang@louisville.edu
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.









