Molecular Weight Determination of Low-Molecular-Weight-Heparins: SEC/MALS vs. SEC/UV-RI
Application Note
Low-molecular-weight heparins (LMWHs) are obtained by fractionation or depolymerization of natural heparins. They are defined as having a mass-average molecular weight of less than 8000 and for which at least 60% of the total weight has a molecular mass less than 8000.
Size-exclusion chromatography (SEC) has been the most common way of measuring the molecular weight and molecular weight distributions of LMWHs by using the two most common detection technologies: Ultraviolet (UV) coupled with refractive index (RI) detection. However, these detectors embody a relative method in order to determine molecular weights, requiring calibration standards. A newer, absolute method involves the use of multi-angle light scattering (MALS), which does not require any standards. The European Pharmacopeia (EP) monograph for LMWH specifies the use of the UV/RI detection method and provides a known calibration standard. Many laboratories around the world have adopted this method.

Figure 1: Examples of UV and RI traces for an LMWH sample.
We previously developed an SEC/MALS method and found it to be very suitable for the analysis of LMWHs. We have recently adopted the UV-RI method described in the EP monograph and compared the molecular weight results generated for LMWH using each detection type. The adopted method uses an Agilent LC-1200 series HPLC, 0.2 M sodium sulphate pH 5.0 mobile phase, Tosoh TSK-gel G2000 SWxl column with Tosoh TSK-gel Guard SWxl, Waters 2487 dual wavelength UV detector, and Wyatt Optilab rEX refractive index detector. For MALS analysis, the UV detector was replaced with a Wyatt miniDAWN TREOS detector; all other method aspects remained the same.
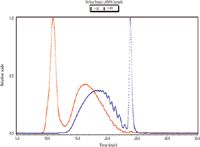
Figure 2: Examples of LS and RI traces for an LMWH sample.
The results indicated that both detection types are suitable and acceptable for the analysis of LMWHs. The molecular weight and distribution results generated using each detection type are comparable. This indicates that a SEC/MALS method could be adopted in place of the SEC/UV-RI method currently required by the EP monograph, and that it would result in less time because it obviates the need for calibration standards.
This note was graciously submitted by Lin Rao and John Beirne of Scientific Protein Laboratories LLC.

Wyatt Technology Corporation
6300 Hollister Avenue, Santa Barbara, California 93117, USA
Tel. +1 (805) 681 9009 fax +1 (805) 681 0123
Website: www.wyatt.com

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










