Modern Trends and Best Practices in Mobile-Phase Selection in Reversed-Phase Chromatography
LCGC Europe
This instalment provides an overview of the modern trends and best practices in mobile-phase selection for reversed-phase liquid chromatography (LC). In particular, we focus on selection criteria and rationales for enhancing analytical performance and ease of preparation.
This instalment provides an overview of the modern trends and best practices in mobile-phase selection for reversed-phase liquid chromatography (LC). In particular, we focus on selection criteria and rationales for enhancing analytical performance and ease of preparation.
Reversed-phase liquid chromatography (LC) is the dominant mode in high performance liquid chromatography (HPLC) for quantitative analysis and is used in ~80% of all HPLC applications (1–3). Reversed-phase LC uses a hydrophobic stationary phase and a polar mobile phase and analytes are retained primarily by hydrophobic interaction. Most analytical methods for purity assessments, quality control, and stability testing of pharmaceuticals employ reversedâphase LC with UV detection because of the excellent precision and reliability of the technique. Another reason for using reversed-phase LC in stabilityâindicating analyses is from a mass balance consideration. The retention in reversed-phase LC is highly correlated to solutes’ log P or partitioning coefficients between water and octanol. Here, the weaker interactive forces of the solutes with the stationary phase give assurance that all impurities within the injected sample are eluted from the column at the end of the purging gradient, thus accounting for all the components in the sample (4).
The role of the mobile phase in controlling retention and selectivity in reversed-phase LC for neutral and ionizable solutes has been discussed extensively in textbooks, and the reader is referred to them for a detailed discussion (1–3). Retention in reversedâphase LC can be predicted by the linear solvent strength model (5) in which the logarithm of the retention factor of the solute is inversely proportional to the percentage of the strong solvent. However, the model is complicated by interactions between solutes and the surrounding polar solvent molecules in the mobile phase, termed the solvophobic interactions model by Horvath and associates (6), and the role played by the absorbed monolayers of the strong solvents to the hydrophobic stationary phase (1). Also, there can be secondary interactions, such as those between basic functional groups undergoing ionic strengthâdependent interactions with free residual silanols present on stationary phase surfaces, which can result in tailed peaks (7).
In this instalment, we present a brief overview of mobile phase fundamentals in reversed-phase LC and describe modern trends in their selection, as summarized in Table 1. The highlighted trends focus on the stability-indicating analysis of pharmaceuticals, including both small molecule drugs and biotherapeutics, increased UV and mass spectrometer (MS) detection sensitivity, and the attainment of symmetrical peak shapes for basic analytes.
Fundamentals of Mobile-Phase Selection
In this section, we describe common selection criteria for the weak (aqueous) and strong (organic) mobile phases in reversed-phase LC and their additives.
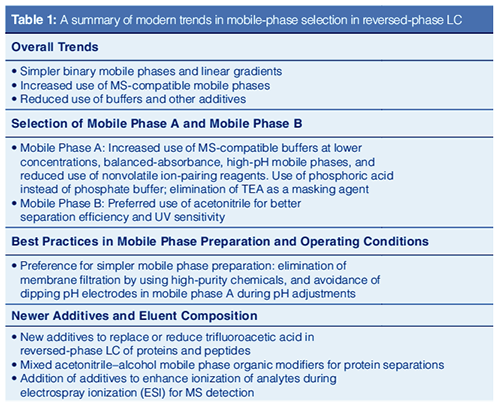
Overall Trends Towards Simpler Mobile Phases: Although the column is the heart of a liquid chromatograph, the mobile phase can be used to manipulate the retention and selectivity of the separation. The column is packed with a high surface area support of variable porosity onto which the stationary phase is bonded. The stationary phase provides retention, and, in conjunction with the mobile phase, differential migration of the solutes. During the method development process, separation of coeluted peaks can be fine-tuned with infinite combinations of mobile phase factors, including solvent type, pH, additives, and operating conditions (such as temperature, flow rate, gradient time).
Paradoxically, innovation in reversed-phase LC centres on new instruments and columns while scant research efforts are placed on the development of newer reagents for mobile phases. Looking at the historical practice of HPLC, there appears to be a trend towards the use of simpler mobile phases for several practical reasons.
First, improved column technologies have reduced the need for mobileâphase additives or buffers to improve peak shapes or column batch-to-batch reproducibility (8). Second, the use of simpler binary solvents with linear gradient segments increases method robustness through reduction of method transfer issues for regulated assays (9). Finally, the rapid emergence of HPLC–MS as a standard technology in highâthroughput screening, in-process control, bioscience research, and clinical diagnostics also favours the use of simpler HPLC methods and mobile phases (10).
Selection of Organic Solvent or Mobile Phase B in ReversedâPhase LC: Mobile phase B is the conventional term for the strong mobile phase (organic solvent) in reversedâphase LC used in pump blending and gradient elution. Similarly, mobile phase A is the weaker, aqueous mobile phase.
Historically, the three most common reversed-phase LC organic solvent choices are acetonitrile, methanol, and tetrahydrofuran. The order of eluotropic strengths are methanol<acetonitrile<tetrahydrofuran (1,2). For instance, a mobile phase of 44% methanol–water was found to have equivalent elution strength of 35% acetonitrile–water or 28% tetrahydrofuran–water for a reference application (11). These three solvents have distinctively different properties in terms of proton acceptor ability, proton donor ability, and dipole interactions (1,11). The selectivity differences of these three solvents in reversed-phase LC can be exploited effectively to develop optimized isocratic separations, as demonstrated by Glajch and associates with the aid of modelling software (12).
Most practitioners prefer acetonitrile because of its strong eluotropic strength, low viscosity (0.37 cP) leading to higher column efficiency, and good UV transparency (to 190 nm). Acetonitrile is an aprotic solvent and is a proton acceptor with capability for π –π interaction (3).
Methanol is a protic solvent and functions as a proton donor or proton acceptor. Methanol is less expensive but yields significantly higher pressure (viscosity of 0.55 cP) than acetonitrile particularly when mixed with water (for example, solution of 50% methanol–water has a viscosity of 1.62 cP) (11). Methanol has substantial UV endâabsorbance below 210 nm.
Tetrahydrofuran is rarely used in reversed-phase LC, despite its strong solubilizing power and eluotropic strength. Toxicity and safety issues as a result of peroxide formation are problems preventing its widespread use except in gel permeation chromatography (GPC). Thus, the choice of mobile phase B is effectively narrowed to acetonitrile and methanol.
Methyl tert-butyl ether can be a substitute for tetrahydrofuran in low concentrations because it has limited miscibility with water and does not form peroxides. Longer chain aliphatic alcohols such as ethanol, propanol, and butanol are not generally used because of their higher viscosities, with notable exceptions in separations of chiral compounds by normalâphase chromatography or in reversedâphase LC of larger proteins. Many publications have appeared in recent years using acetonitrile mixed with n- or i-propanol, or even n-butanol, which seems to be of benefit to improve mass recovery of some monoclonal antibodies (mAbs). Dimethyl sulfoxide has strong solubilizing power as a diluent but suffers from a very high viscosity and UV cutoffs (3), as well as incompatibility with specific polymeric materials (such as PEEK) used in some HPLC instruments.
The Use of Mobile Phase B Containing Water and Additives: One often sees HPLC methods with a stipulated mobile phase B such as 95% acetonitrile in water. The rationale is to help mixing efficiency by making the two mobile phases more similar in viscosity and surface tension. The cons are the reduction of solvent strength of mobile phase B and an extra step in its preparation. With the improvements of modern pumps and online mixers, this additional step appears to be less beneficial.
There is also a common practice to use the same levels of additives in both mobile phases A and B, such as 0.1% trifluoroacetic acid in acetonitrile when using 0.1% trifluoroacetic acid (TFA) in water as mobile phase A. Technically, there are no perceivable differences between 100% acetonitrile or 0.1% trifluoroacetic acid in acetonitrile for separation reproducibility or elution order in reversed-phase LC except for reducing gradient shifts with UV detection at low wavelengths (to be covered in a later section).
Mobile Phase A: pH Modifiers and Buffers: As noted above, the weaker mobile phase in reversedâphase LC is termed mobile phase A which consists largely of water, often containing small amounts of modifiers, buffers, or salts for controlling pH and ionic strength. Pure water can be used for the separation of neutral molecules.
In pharmaceutical analysis, most drugs are ionizable, that is, acidic, basic, or zwitterionic. Thus, the pH of mobile phase A must be controlled, as it can have a dramatic effect on solute retention. Ionizable solutes can exist in ionized or nonionized forms depending on the mobile phase pH, and the ionized forms have significantly lower retention than the non-ionized forms in reversed-phase LC.
Table 2 lists the common mobile phase additives with their respective pKa and UV cutoffs. Volatile buffers labelled with an asterisk are used for LC–MS applications. The additives can be an acidifying or basifying agent or a buffer by the addition of its respective conjugate salt.
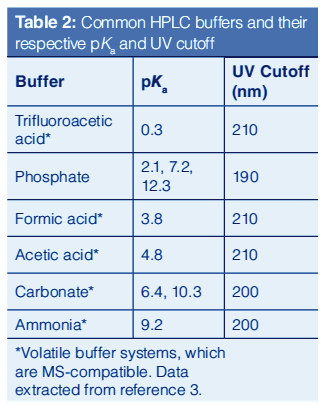
Acidic Additives: An acidic pH of 2–4 is used for most pharmaceutical applications. The low pH suppresses the ionization of weakly acidic analytes, leading to higher retention (3). Basic analytes are ionized at low pH, though most basic drugs have sufficient hydrophobicity for adequate reversed-phase LC retention. An acidic pH also suppresses the ionization of acidic residual silanols to Si-O-ions, which can cause secondary interactions with basic analytes.
Commonly used acids are trifluoroacetic acid, formic acid, and acetic acid at concentrations of 0.05 to 0.1% v/v. In aqueous solutions, the pH values are: 0.1% trifluoroacetic acid (2.1), 0.1% formic acid (2.8), and 0.1% acetic acid (3.2). These simple mobile phases at 0.1% v/v are prepared by pipetting 1.0 mL of the acid into 1 L of purified water and can be used directly without further filtration. They are often used in LC–MS applications though their low ionic strengths may yield poor peak shapes for very basic drugs (7,13).
The use of 0.1% phosphoric acid appears to be under-utilized because there is a traditional preference for phosphate buffer. It is readily prepared and is transparent down to 200 nm. This additive is useful for purity methods of raw materials or reagents using more universal detection at low UV.
Buffers: Buffers are required to tightly control the pH of mobile phase A for critical assays. According to the Henderson-Hasselbalch equation, buffers are most effective within ±1.0 pH units from their pKa values (1,11). A buffer is prepared by mixing a weak acid with the salt of its conjugate base (or a weak base with the salt of its conjugate acid).
Historically, the most common buffer used in HPLC is phosphate. Because phosphoric acid has three ionizable hydrogens, phosphate buffers make effective buffer systems at pH values around 2, 7, and 10, respectively. It is UV-transparent to 200 nm, but it is not volatile, and thereby not MS-compatible. It also suffers from poor solubility in acetonitrile, particularly at high concentration (such as 50 mM) causing precipitation problems during pump blending. Changing mobile phase B to 85% acetonitrile in water or methanol can alleviate this issue.
Buffers of ammonium salts of volatile acids are used for the development of MS-compatible HPLC methods. A common MS-compatible buffer system is 20 mM ammonium formate adjusted to pH 3.7 with formic acid. This mobile phase appears to yield excellent peak shapes for most basic drugs and peptides on modern columns, and appears to help retention of many basic or zwitterionic analytes, particularly as sample load increases (7,14). Higher concentrations can be used effectively to increase retention in reversed-phase LC by the “salting out” effect or the Le Chatelier’s principle to drive solutes into the hydrophobic stationary phase (1,11).
Basic Mobile Phases: Before the 1990s, silica-based columns could not be used with high pH mobile phases, because of the dissolution of silica support at pH values >8. The development of hybrid particles and improved bonding chemistries for silica particles has extended the usable pH range of these specific columns to a range of 1–12 (2–10 at column temperatures >40 °C) (3,15–16). Typical high pH mobile phases are 0.05–0.1% ammonia, and 10–25 mM ammonium carbonate, or phosphate buffers. Comparisons of mobile-phase buffers at elevated pH conditions revealed significant effects on silica solubility and expectations for stability under high pH and temperature conditions (16). Important observations were that ammonium hydroxide is perhaps the safest choice and has good compatibility with ESIâMS detection. The advantages of using high pH separations include higher retention of water-soluble bases (for example, for the analysis of opioid drugs, which traditionally require ion-pairing chromatography), better peak shapes for basic analytes, and MS-compatibility (15). The disadvantages are the instability of some drugs in basic mobile phases and potential method robustness issues if the mobile phase pH is close to the pKa values of the basic analytes (for example, most amines have pKa values in the range of 8–10) (3,15).
Figure 1 shows a chromatogram of a test mixture containing two active pharmaceutical ingredients (APIs) spiked with process impurities and degradants for an MS-compatible stability-indicating assay of a combinational drug product (3). Note the excellent peak shapes of all peaks under high-pH conditions without any peak splitting. The disadvantage of this method is its sensitivity to mobile phase pH, which must be kept between 9.10 to 9.15 to prevent any peak coelution (3,15).
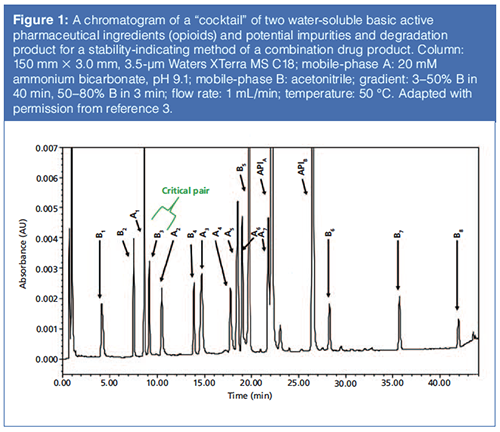
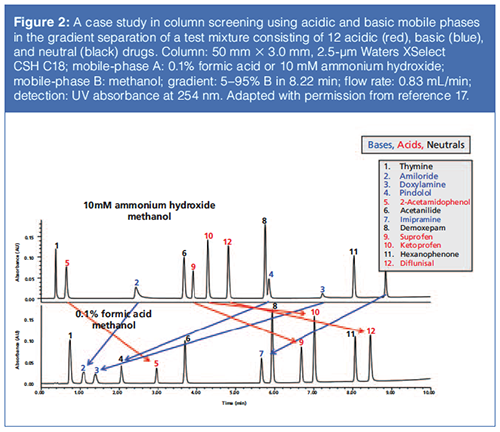
Figure 2 further illustrates the effect of mobile-phase pH to dramatically alter the retention and elution orders of acidic and basic drugs (17). The use of column screening using mobile phase A at both acidic and basic pH has become a common practice in many laboratories for high-throughput screening and development of critical methods (3, 8–10).
Reduced Usage of SilanolâMasking, Ion-Pairing and Chaotropic Reagents: Prior to the 1990s, the primary complaint against silica-based HPLC columns was the difficulty in method transfer for quality control applications caused by batch-to-batch differences of raw silica support or bonded phase chemistry. The main cause of this non-reproducibility was the presence of active silanols, which are activated by metallic impurities (1,3). At that time, many bonded phases had such acidic silanols that basic solutes would not elute without the use of silanol-masking reagent additives in the mobile phase (such as triethylamine). Triethylamine was frequently used in drug analysis to yield better peak shapes and acceptable batch-toâbatch reproducibility in regulated testing (18).
Today, the popularity of high-purity silica supports, and the elimination of metallic impurities in the base silica, leads to reduced silanophilic activities and much improved batchâto-batch reproducibility of bonded phases. The use of triethylamine as an additive has generally been abandoned for several reasons. First, triethylamine may permanently alter the selectivity of the column. Second, it would overwhelm all MS signals in the positive ionization mode. Third, new column technologies using charged surface and other approaches provide a better solution to improve peak shape of basic analytes.
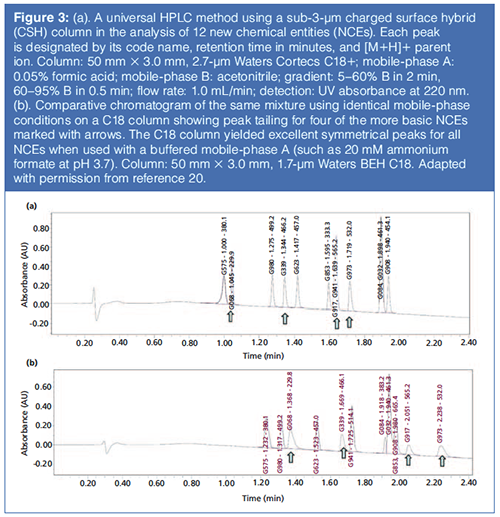
A fairly recent innovation has been the bonding of a positive charge (for example, an amine) to the surface of bonded phases (reviewed in Nawrocki, 30), such as the case of charged surface hybrids (CSH) introduced by Waters in 2010 (13). These columns yield excellent peak shapes for highly basic drugs even in mobile phases with low ionic strengths (such as 0.1% formic acid) as illustrated in Figures 3(a) and 3(b) showing comparative chromatograms of a test mix of 12 new chemical entities (NCEs) using a low ionic strength mobile phase A of 0.1% formic acid on columns packed with a CSH compared to those on a hybrid bonded phase (19,20). The CSH C18+ column shows excellent peak shapes for all NCEs while the other column displays considerable peak tailing for four of the more basic NCEs.
Another approach is to use an advanced coating technique to reduce the activities of the residual silanols. An added benefit of this approach is to yield columns usable at high pH (such as the Agilent Poroshell HPH-C18) (20).
Ion-Pairing and Chaotropic Reagents: Ion-pairing reagents are detergent-like molecules added to mobile phase A to provide retention of acidic or basic analytes (1). Long-chain alkyl sulfonates (C5 to C12) combine with basic solutes under acidic pH conditions to form neutral “ion-pairs” that are retained in reversed-phase LC. Retention is proportional to the length of the hydrophobic chain of the ionâpairing agent and its concentration of the ion-pairing reagent. Note that trifluoroacetic acid has some ion-pairing capability and is commonly used in reversedâphase LC of proteins and peptides. The use of ion-pairing reagents has decreased somewhat in recent years, because of issues such as lower column efficiencies, slow column equilibration, difficulties in performing gradient analysis, and the lack of MS-compatibility (1,2). Also, several companies have produced specialty surface-modified HPLC columns that reduce the need for ion-pairing reagents, by resolving the problem of “phase collapse” or “pore dewetting” at low or no organic solvent reversed-phase LC mobile phase conditions (3). Such materials can retain highly polar bases and acids, with less reliance on hydrophobic counterion additives, ensuring better compatibility with MS detection, and faster re-equilibration between separations.
Another class of additives used to increase retention and selectivity of basic analytes is inorganic chaotropic agents (such as PF6-, BF4-, ClO4- ions) that form neutral ion pairs under acidic conditions in reversed-phase LC (1,2). Chaotropes are better suited for gradient analysis and can produce lesser baseline shifts, but are not MS-compatible.
Note that availability of highâpHâcompatible volatile mobile phases (such as ammonium hydroxide), with suitable silicaâbased or hybrid columns, can offer a compelling alternative MS-compatible approach for the analysis of waterâsoluble basic drugs, and thereby reduce the need for ionâpairing or chaotropic reagents for such separations (15).
Other Mobile Phase Additives for Chiral Separations and Ion Chromatography: It is possible to use a chiral additive as a pairing reagent to form a diastereomer, enabling chiral separations to be achieved on conventional reversedâphase columns (1). However, the availability of versatile and highly efficient chiral stationary phases (such as immobilized and chemically-modified polysaccharides) has reduced the need for these indirect methods for chiral separations (1–3).
Ion chromatography sometimes employs a mobile phase additive (such as 3 mM sodium phthalate) to perform ion chromatography by conventional HPLC–UV equipment with indirect photometric detection of the displaced complex (21). However, the widespread availability of improved ion chromatography instruments with suppressed conductivity or MS detection has eliminated most of these approaches with limited applicability.
The Use of Balanced Absorbance Mobile Phases: During gradient analysis using mobile phases with UV detection at low wavelengths (such as <230 nm using MS-compatible mobile phases), substantial gradient baseline shifts are observed as a result of an imbalance of absorbance and refractive indices of mobile phases A and B. The magnitude and direction of the baseline shift are strongly dependent on detector flow cell design, wavelength, and mobile phase composition. These imbalances can also be attributed to the chromophoric shifts of the UV spectra of the additives (such as carboxylic acids) in the two very different solvent environments (3). The differences in the apparent absorbance (absorbance detector signal) and the inadequate mixing of mobile phase A and B solvents can often also be observed as a periodic (sinusoidal) baseline disturbance. Again, the magnitude of such baseline disturbances is a complex function of the online mixer design, volume, and apparent absorbance differences between pure mobile phase A and mobile phase B.
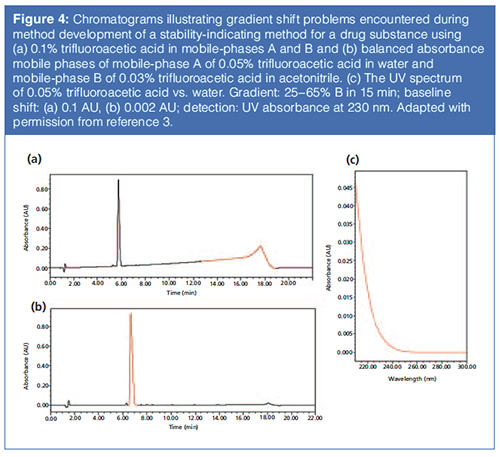
Figure 4(a) illustrates the gradient baseline shift observed during method development of a stability-indicating method for an NCE using mobile phase A (0.1% trifluoroacetic acid) in water and mobile phase B (0.1% trifluoroacetic acid in acetonitrile) (3). Because of the absorbance of trifluoroacetic acid in the far UV region (200–230 nm), significant baseline shift (~0.1 AU at 230 nm) was observed. These baseline problems were substantially reduced by lowering the concentration of trifluoroacetic acid to 0.05% (see the UV spectrum in Figure 4[b]). Further reduction of the gradient shift to 0.002 AU at 230 nm was achieved by adjusting the concentration of trifluoroacetic acid in mobile phase B to 0.03%, which has a similar apparent absorbance at 230 nm with 0.05% trifluoroacetic acid in water (Figure 4[c]).
When using UV–vis absorbance detectors, suitable adjustments of baseline properties, as described above, are required to achieve the highest levels of UV sensitivity.
Modern Trends in Mobile Phase Preparation
Modern trends in mobile phase preparation include the use of lower buffer concentrations, the elimination of the filtration process, and pH adjustment procedure for buffers.
Lower Buffer Concentrations in Mobile Phase A: Although 50 mM buffers are specified in many older methods, the modern trend is to use lower buffer strengths (such as 5–20 mM), which provide sufficient buffering capacity in most applications (3). Lately, the use of modern columns with lower residual acidic silanol activities, or CSH materials, has allowed the use of simplified mobile phase A with low ionic strength, such as 0.1% formic acid, while permitting good peak shapes for highly basic analytes (11,13,20)
Elimination of the Filtration Process: Many laboratories have eliminated the filtration process with 0.2- or 0.5-µm membrane filters by using high-purity reagents (such as 99.995% ammonium formate from Aldrich, Cat# 516961), and HPLCâgrade solvents and water (22). The internal filters in most HPLC pumps (replaced during preventive maintenance programs) appear to be adequate to allow successful routine HPLC operation without filtering in most laboratories. This elimination of the filtration step can reduce potential mobile phase contaminations from the filtration process. Exceptions are mobile phases containing ion-pairing reagents and high-concentrations of salts, or when high-purity reagents are not readily available.
Similarly, the use of vacuum filtration for dissolved gas reduction in mobile phases has been largely superseded by inline vacuum degassers, which are standard on most modern HPLC systems.
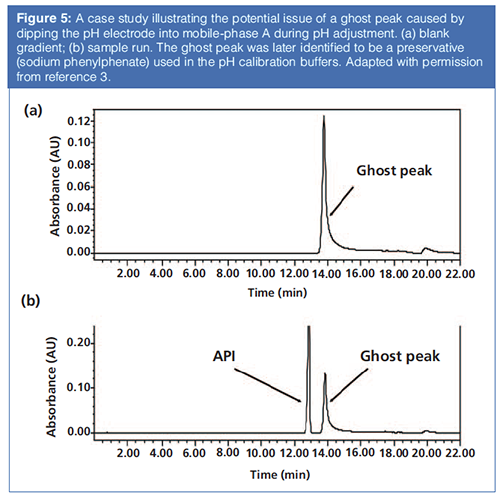
Adjusting the pH of Mobile Phase A: Many analysts prepare buffers by dipping the pH electrode directly in mobile phase A and titrating the solution until the designated pH is reached. This practice can be problematic because trace contaminants adhering to the electrodes (such as preservatives from the pH calibration buffers to allow room temperature storage) can cause substantial ghost peaks in gradient elution (see the example in Figure 5). To prevent such contamination, the analyst should dispense a small amount of mobile phase A into a vial (such as a scintillation vial) to check the pH and continue this iteration until the correct pH is reached. The content of the vial should not be poured back into mobile phase A (3). With suitable care, the correct composition of mobile phase A can be maintained, with minimal risk of introducing contaminants that can readily appear in high sensitivity analyses.
Modern Trends in Gradient Methods
Modern trends in gradient methods include the use of simple binary mobile phases (such as 0.1% formic acid or 0.1% ammonia) with highâefficiency columns packed with superficially porous particles and low-silanophilic bonded phases (8). Robust stability-indicating methods for very complex pharmaceuticals can be accomplished by using multisegment binary linear gradients (23). Although quaternary pumps are widely used for method development, quality control methods that use ternary or quaternary mobile phases are rarely encountered, because of the difficulties with method reproducibility across different laboratories or equipment platforms. For that matter, the use of concave or convex gradients is strongly discouraged, even though they are available in some systems. Method transfer can be less problematic with simplified methods using easily prepared binary mobile phases.
Comments on the Stability of Mobile Phases
There are no universal guidelines or scientific data on mobile phase stability after preparation. Obviously, fresh mobile phases can be prepared daily as needed, though this time-consuming process is neither a necessary or “green” practice. The shelf life of the mobile phase is difficult to generalize and is dependent on mobile phase composition, pH, storage container, storage conditions, and the sensitivity of the separation to small changes in mobile-phase composition. The potentials for growth of microorganisms (bacteria, algae, molds) poses the greatest risks to system contamination and column longevity. The use of a scavenger column (for example, C18, 33 × 4.6 mm, 10-µm) (24) can provide excellent protection against mobile-phase-borne particulates and contaminants. However, it does add system dwell volume and is rarely used in today’s laboratories.
A common practice is to date all mobile phases and use aqueous buffers for one week, simple acidified water (such as 0.1% formic acid or trifluoroacetic acid) for 1–2 months, and organic solvents for at least 3 months. For weakly acidic buffered mobile phases or buffers at near neutral pH, storage of a concentrate (10–50×) can be useful. Depending on the specific buffer, refrigeration (5 °C) can permit the use of the concentrate for many months. This approach has worked well for acetate and formate buffers. In such cases, periodic checks of the pH and blank injections can verify the integrity and cleanliness of the mobile phase preparations. There may be standard operating procedures in some laboratories that stipulate reagent shelf life based on historical data. In any case, labelling of the preparation date is encouraged and required in many regulated laboratories.
Newer Additives for ReversedâPhase LC of Proteins and Peptides
Separation of proteins and peptides is a challenging application for HPLC requiring bonded phases with low silanophilic activity, specific mobileâphase additives to allow detection at low UV wavelengths (210–220 nm), and higher column temperatures (>60 °C) to improve peak shapes, and mass recovery (2,3).
The traditional mobile phase A for protein and peptide separations is 0.1% trifluoroacetic acid, which has ion-pairing capability for better peak shapes and offers reasonable UV sensitivity at 210–220 nm for peptide bonds. Although trifluoroacetic acid is considered MS-compatible from a volatility standpoint, MS sensitivity is seriously compromised as a result of ion suppression when trifluoroacetic acid is used during the electrospray ionization (ESI) process. Trifluoroacetic acid is an excellent ion pair reagent with primary ϵ-amino lysine group, as well as the N-terminal α-amino group. In addition to supplying a strongly acidic modifier for reversed-phase LC separations, trifluoroacetic acid shows a favourable increment in retention of acidic peptides, because of the formation of an N-terminal amino ion pair, which is notably absent in formic acid mobile phases. Nevertheless, MS sensitivity will be impaired for protein and peptide detection using trifluoroacetic acid, when compared to formic acid alone, or compared to mixtures of trifluoroacetic acid and formic acid. The latter approach has some popularity, with the use of lowered concentration of trifluoroacetic acid in mobile phases A and B, in the presence of equal or greater concentration of formic acid to act
as a competitor to trifluoroacetic acid, during gas phase exchange in the ESI process (25,26).
Recent research by Boyes and associates has uncovered two promising additives that deliver a good compromise of performance for peak shape and MS sensitivity: difluoroacetic acid and 3-fluoropropionic acid (26). Figure 6 illustrates the comparative performance of four mobile phase additives (formic acid, ammonium formate/formic acid, trifluoroacetic acid, and difluoroacetic acid) in the HPLC–UV–MS analysis of a mixture of five peptides. The HPLC–UV and total ion chromatogram (TIC) data indicate that difluoroacetic acid offers excellent performance in peak shape and sensitivity under UV and MS detection.
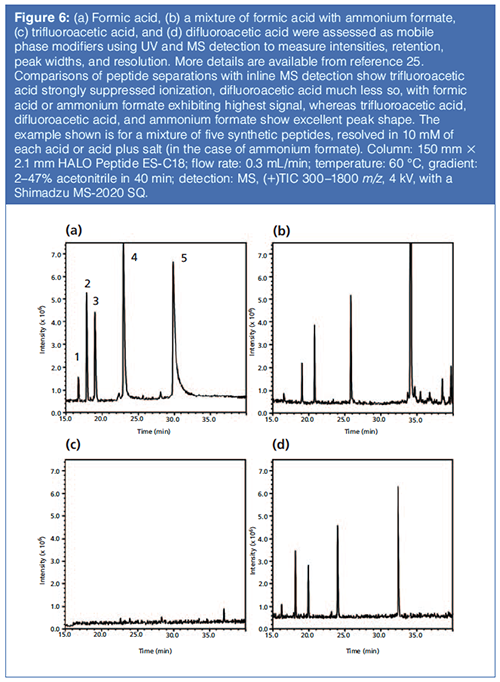
Figure 7 shows comparative chromatograms in a peptide mapping application showing excellent performance in peak shape and MS sensitivity for 10 mM difluoroacetic acid compared to those for formic acid, trifluoroacetic acid, and a mixture of formic acid and ammonium formate. Figure 8 shows an example of a protein separation using trifluoroacetic acid compared to difluoroacetic acid and formic acid in mobile phase A.
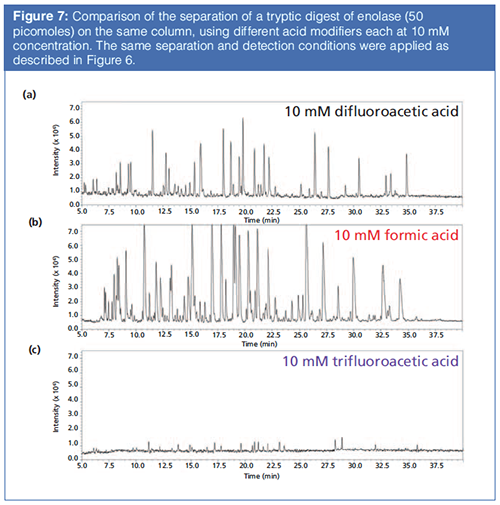
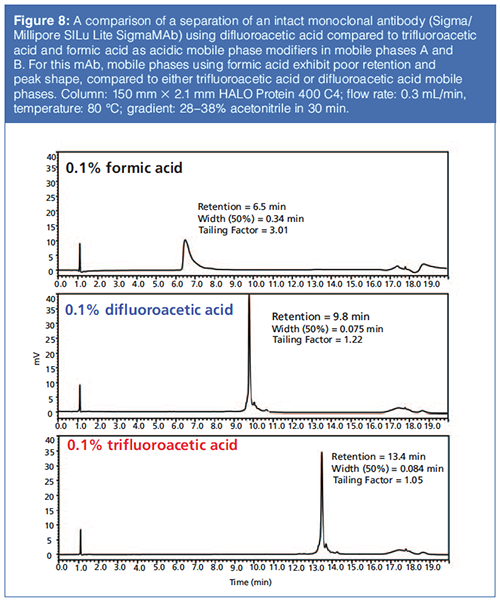
Anecdotal evidence suggests that the advantages of difluoroacetic acid as a replacement are more apparent for ESI signal intensities of peptides and smaller proteins, compared to larger proteins up to the size of immunoglobins. Regardless, difluoroacetic acid is much easier to flush from LC systems than trifluoroacetic acid, which requires special and time-consuming procedures to completely flush out of LC–MS systems. Basically, trifluoroacetic acid appears more “sticky” towards components in the LC system than difluoroacetic acid. Over more than six years of use, difluoroacetic acid has shown no negative effects on instrument performance in standard or capillary column separations.
Another approach that has emerged is the use of additives to mobile phase A to increase, or rescue, ionization efficiency lost to trifluoroacetic acid ionization suppression. Dimethyl sulfoxide has been suggested as an additive for improving MS detection in high complexity or high sensitivity proteomic sample LC–MS analyses (27,28). Several confirmatory examples are described for proteomics analysis, although some concerns remain about the long-term effects on LC–MS components, and the tendency of dimethyl sulfoxide (or impurities or break-down products) to accumulate in MS systems over time. Various weak “Brønsted” base compounds, shown to act as “supercharging agents”, have been studied as mobile phase A additives to improve MS signal intensity of proteins resolved in reversedâphase LC, using trifluoroacetic acid modified mobile phase A (29). The addition of such compounds does not hinder effective LC performance and has been shown to restore signal intensities heavily suppressed by trifluoroacetic acid. The effect of these additives on MS system stability and maintenance are still unknown.
Summary
In this instalment, we present an overview of modern trends and best practices in mobile-phase selection and preparation in reversed-phase LC, with a focus on pharmaceutical applications and increasing peak shape and detection performance by UV and MS. These trends include the use of simple acidic mobile phase A or low-concentration MS-compatible buffers with acetonitrile or methanol in binary mode, using broad linear gradient or multi-segment gradient methods for complex separation, the elimination of the filtration process, and many less relevant mobile phase additives. Newer additives (difluoroacetic acid and 3-fluoropropionic acid) as alternatives to trifluoroacetic acid for an increased MS sensitivity are suggested.
Acknowledgements
The authors thank the following colleagues for their review of the manuscript: Matt Mullaney, Tuty Norashikin of MSD International GMbH, Mike Shifflet of J&J Consumer Healthcare, M. Farooq Wahab of U. Texas at Arlington, Patrick Cronan of Agilent Technologies, Brian R. Holder of Merck, He Meng of Sanofi, and Justin Godinho at AMT.
References
- L.R. Snyder and J.J. Kirkland, Introduction to Modern Liquid Chromatography (Wiley, Hoboken, New Jersey, USA, 3rd Ed., 2010), Chapters 2, 6, and 7.
- Y.V. Kazakevich and R. LoBrutto, Eds., HPLC for Pharmaceutical Scientists (Wiley, Hoboken, New Jersey, USA, 2007), Chapter 4.
- M.W. Dong, Modern HPLC for Practicing Scientists (Wiley, Hoboken, New Jersey, USA, 2006), Chapters 1, 2, 5, 6, 8, and 10.
- M.W. Dong, LCGC Europe 26(7), 393–399 (2013).
- L.R. Snyder and J.W. Dolan, HighâPerformance Gradient Elution: The Practical Application of the Linear-Solvent-Strength Model (Wiley, Hoboken, New Jersey, USA, 2006).
- W.R. Melander and C. Horváth, in HPLC, Advances and Perspectives Vol. 2, C. Horváth, Ed. (Academic Press, New York, USA, 1980).
- D.V. McCalley, J. Chromatogr. A 1217(6), 858–880 (2010).
- D. Guillarme and M.W. Dong, Amer. Pharm. Rev. 16(4), 36–43 (2013).
- M.W. Dong, LCGC Europe 26(8), 455–461 (2013).
- M. Wong, B. Murphy, J.H. Pease, and M.W. Dong, LCGC Europe 28(6), 343–352 (2015).
- K. Schug and T. Taylor, The Essential CHROMacademy Guide: Mobile Phase Optimization Strategies for Reversed Phase HPLC, Webcast, http://www.chromacademy.com/essential_guide_webcast/mobile_phase_optimization_strategies_for_reversed_phase_hplc/mobile_phase_optimization_strategies_for_reversed_phase_hplc.pdf (accessed on 27/6/2018).
- J.L. Glajch, J.J. Kirkland, K.M. Squire, and J.M. Minor, J. Chromatogr. 199, 57 (1980).
- K.J. Fountain, H.B. Hewitson, P.C. Iraneta, and D. Morrison, Waters Corporation, Milford, Massachusetts, USA, 720003720EN, Sep. 2010.
- D. Johnson, B.E. Boyes, and R.C. Orlando, J. Biomol. Technology 24, 187–197 (2013).
- M.W. Dong, G. Miller, and R. Paul, J. Chromatogr. 987, 283–290 (2003).
- J.J. Kirkland, M.A. van Straten, and H.A. Claessens, J. Chromatogr. A 691, 3–19 (1995).
- J.E. Turner, A Chemistry Focused Approach to Column Screening, HPLC Method Development Workshop, Parsippany, New Jersey, USA, 13 June 2018.
- D.W. Hill and A.J. Kind, J. Anal. Toxicol. 19, 233–242 (1994).
- M.W. Dong, LCGC Europe 29(6), 328–337 (2016).
- M.W. Dong, LCGC Europe 29(9), 516–527 (2016).
- M.W. Dong and R.M. Woods, LCGC North Am. 34(10), 792–797 (2016).
- M.W. Dong, LCGC Europe 27(8), 415–419 (2014).
- M.W. Dong, D. Guillarme, S. Fekete, R. Rangelova, J. Richards, D. Prudhomme, and N.P. Chetwyn, LCGC North Am. 32(11), 868–876 (2014).
- M.W. Dong, J.R. Gant, and P.A. Perrone, LCGC 3, 786–795 (1985).
- B. Boyes, S. Schuster, J.J. Kirkland, B. Wagner, B. Libert, and J. DeStefano, Proceedings ASMS, St. Louis, Missouri, USA (2015), ID 234745
- B.E. Boyes, W. Miles and B. Libert, J. Biomol. Technology (2018), in press.
- F.E. Kuhlman, A. Apffel, S.M. Fischer, G. Goldberg, and P.C. Goodley, J. Am. Soc. Mass Spectrom. 6, 1221–1225 (1995).
- H. Hahne, F. Pachl, B. Ruprecht, S.K. Maier, S. Klaeger, D. Helm, G. Médard, M. Wilm, S. Lemeer, and B. Kuster, Nat. Methods 10(10), 989–992 (2013).
- M. Nshanian, R. Lakshmanan, H. Chen, R.R. Ogorzalek Loo, and J.A. Loo, Int. J. Mass Spectrom. 427, 157–164 (2018).
- J. Nawrock, J. Chromatogr. A 779(1), 29–71 (1997).
Barry E. Boyes is the Vice President of R&D at Advanced Materials Technologies (AMT) in Wilmington, Delaware, USA. Barry completed a B.Sc. in biochemistry at the University of Alberta, then M.Sc. and Ph.D. degrees in neuroscience at UBC (Vancouver, Canada). Barry has worked at DuPont Central Research, Rockland Technologies, HP/Agilent Technologies, and Smiths Detection. In 2009, Barry returned to developing separations technologies at AMT, including the refined superficially porous particles pioneered by Jack Kirkland. Barry has published more than 85 peer-reviewed papers, reviews, patents, and applications. He was an adjunct professor of chemistry at the University of Georgia (Athens, Georgia, USA), and continues a long-term collaboration with Professor Ron Orlando at the Complex Carbohydrate Research Center in Athens.
Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, method improvements, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, Research Fellow at Purdue Pharma, and Senior Staff Scientist at Applied Biosystems/PerkinElmer. He holds a Ph.D. in analytical chemistry from City University of New York, USA. He has more than 100 publications and a best-selling book in chromatography. He is an editorial advisory board member of LCGC North America and the Chinese American Chromatography Association. Direct correspondence to: LCGCedit@ubm.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.