Microextraction and Its Application to Forensic Toxicology Analysis
This instalment describes several commonly used microextraction sample preparation techniques and their applications to forensic toxicology analysis. Solid-phase microextraction (SPME), microextraction by packed sorbent (MEPS), and different types of liquid-based microextraction (LPME), including single‑drop microextraction (SDME), hollow-fibre supported LPME, three-phase LPME, and dispersive liquid–liquid microextraction (DLLME), are discussed. Examples of application of these techniques to determine illicit drugs and drugs of abuse from various biological specimens are provided as well.
Yi He, John Jay College of Criminal Justice, City University of New York, New York, USA
This instalment describes several commonly used microextraction sample preparation techniques and their applications to forensic toxicology analysis. Solid-phase microextraction (SPME), microextraction by packed sorbent (MEPS), and different types of liquid-based microextraction (LPME), including singleâdrop microextraction (SDME), hollow-fibre supported LPME, three-phase LPME, and dispersive liquid–liquid microextraction (DLLME), are discussed. Examples of application of these techniques to determine illicit drugs and drugs of abuse from various biological specimens are provided as well.
Forensic toxicology analysis mainly deals with qualitative and quantitative determination of drugs and their metabolites, poisons, and other related compounds in biological specimens to aid medical or legal investigation of death, poisoning, and drug use (1). Specimens used in forensic toxicology analysis usually include urine, blood (plasma, serum), tissues, oral fluid, hair, vitreous humour, and so on. They all have complicated sample matrices. Concentrations of the target analytes, for example, the illicit drugs and their metabolites, are often present at low levels and require enrichment of analytes during sample preparation. Another important issue to consider is the compatibility of the target compounds with the analytical instrument. For example, derivatization may be needed when using gas chromatography (GC) to analyze drugs with polar functional groups. Sample preparation, therefore, plays a very important role in forensic toxicology analysis, which is achieved through cleaning up the sample matrix, isolating the target compounds, enriching analyte concentrations, and converting the chemical to a form compatible with an available analytical instrument.
Many techniques, including liquid–liquid extraction (LLE), solidâphase extraction (SPE), supercritical fluid extraction (SFE), accelerated solvent extraction (ASE), and microextraction methods, are used in forensic toxicology laboratories. Among them, LLE and SPE are the two most commonly used methods. LLE is simple and effective, but uses significant amounts of high purity organic solvent, resulting in cost and environmental concerns. SPE is the workhorse in both commercial and research laboratories. Standard operation procedures and commercial cartridges designed for specific drug analysis are available. SPE involves multistep operation and uses certain amount of solvent. Microextraction methods were developed as alternative choices to conventional techniques to simplify extraction procedure and reduce, or totally eliminate, the use of organic solvent.
Microextraction
Since the introduction of solid-phase microextraction (SPME) in 1990 (2), the microextraction technique has recently grown at a fast speed and has found many applications in various fields including forensic toxicology analysis. This article reviews the following microextraction methods: SPME, microextraction by packed sorbent (MEPS), and different types of liquid-phase microextraction (LPME), including single-drop microextraction (SDME), hollow-fibre supported LPME, three-phase LPME, and dispersive liquid–liquid microextraction (DLLME). Their applications to analyzing compounds with legal and law enforcement interest are presented as well.
Microextraction methods in general can be classified into two big categories depending on whether the exaction is based on sorbent or solvent. SPME is an example of sorbent extraction. Analytes are isolated and enriched by a thin layer of extraction material - such as polydimethylsiloxane (PDMS) - coated on the extraction fibre. The SPME fibre is immersed in sample solution or hangs in the headspace of a container to perform direct immersion extraction or headspace extraction. SPME is most commonly connected with GC because the SPME device can be conveniently used as an injection syringe and no special interface is required. Although SPME can be combined with liquid chromatography (LC), a specifically designed commercially available injector is needed for solvent desorption of analytes from the extraction fibre. Fewer choices of extraction fibre are available as compared with those for GC application.
Another sorbent-based microextraction technique is MEPS (3,4). MEPS is viewed as miniaturized SPE. However, there are a lot of differences compared with the conventional SPE. In MEPS, the extraction cartridge is integrated with a chromatographic syringe and can be used repeatedly more than 100 times, rather than a separate oneâtime use cartridge in SPE. MEPS reduces the volume of solvent and sample needed to millilitre level. By using different packing materials, MEPS is flexible to perform reversedâphase, normal-phase, mixedâmode, and ion-exchange chemistry and can be connected online with GC, high performance liquid chromatography (HPLC), and capillary electrochromatography (CEC) (5). MEPS can also be fully automated.
Various formats of solvent-based microextraction have been developed in the past 20 years. Extraction can be carried out using a single droplet of solvent hanging from the tip of a chromatography syringe, the outlet of a capillary tube, or a sample vial (6). To improve the stability of the solvent drop during single-droplet microextraction (SDME), the extraction drop can be held inside a small segment of hollow fibre, which is termed as hollow-fibre protected (or based) microextraction.
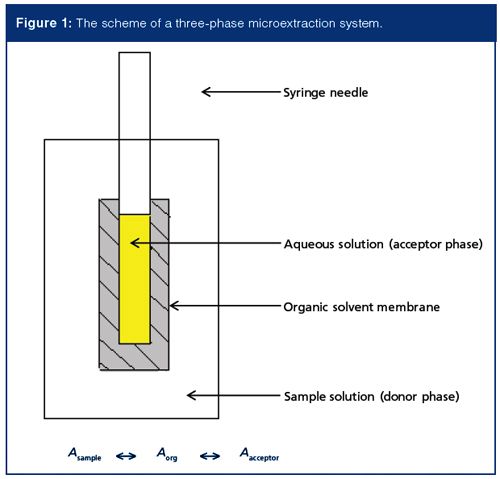
In addition to regular two-phase extraction (sample phase and solvent phase), three-phase extraction, in which a back-extraction solvent is included can be carried out by SDME or hollow fibre–based microextraction. Figure 1 illustrates the scheme of a hollow fibre–based three-phase microextraction. The three phases are sample solution (donor phase), a thin layer of organic phase, and backâextraction solution (acceptor phase). This method integrates extraction and back-extraction in a single step. It is especially suitable for analyzing ionizable compounds whose chemical form is sensitive to pH change. Many drugs and their metabolites that are of forensic toxicology interest contain acidic or basic functional groups, so threeâphase microextraction is a method of choice for these types of compounds. By manipulating the pH of the sample solution and the backâextraction solution, the analyte first presents as its undissociated form and is extracted by organic solvent, which is usually present as a thin solvent film, and then is backâextracted from the solvent film to an acceptor phase. The pH of the acceptor phase promotes ionization of the analyte and keeps analytes in the acceptor phase. Because the analytes are extracted twice in this process, the enrichment factor is usually high. For example, 500- and 730-fold enrichment was easily obtained for the extraction of basic amphetamine and methamphetamine using a threeâphase microextraction system (7). In addition, method selectivity would be enhanced as well because the protonation and deprotonation process occur at two different pH values specific to target analyte.
DLLME is another method of choice for forensic toxicology analysis. The significant advantage of this method is its fast extraction speed. The entire extraction can be completed in a few minutes. The original DLLME procedure introduces organic solvent together with a dispersing reagent into an aqueous sample (8). A cloudy mixture is produced as solvent is dispersed as fine droplets into aqueous matrix, which generates a large contact surface area between these two phases and promotes rapid mass transfer of analytes from sample solution to organic phase. After extraction, the mixture is centrifuged for phase separation and the organic phase is collected for chromatographic analysis. With the development of this technique, in addition to using dispersing reagent, other approaches, such as ultrasound dispersing, vortexing, changing temperature, or adding surfactant, can also be used to generate the emulsion and perform DLLME (9–11). Figure 2 shows the flow chart of a general process for DLLME.
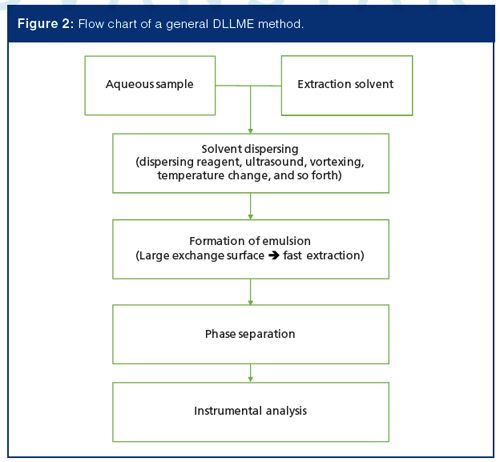
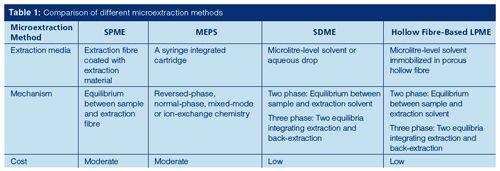
Table 1 summarizes and compares the features of the aforementioned microextraction methods.
Instruments Combined with Microextraction Methods
Microextraction methods can easily be combined with various chemical analysis instruments. Organic compounds are commonly analyzed by GC or HPLC after microextraction, even though other techniques such as capillary electrophoresis (CE) were also used (12–16).
Gas chromatography–mass spectrometry (GC–MS) was regarded as the gold standard in forensic toxicology laboratories because of its excellent sensitivity and selectivity. However, many compounds of forensic toxicology interests are not suitable for direct analysis. Derivatization is needed when choosing microextraction methods before GC analysis. Derivatization can be performed pre-extraction, post-extraction, or in situ, in which derivatization reactions are performed simultaneously with extraction (1,6).
With the development of instrument technology, especially in liquid chromatography–mass spectrometry (LC–MS), more and more toxicologists adapted LC–MS in their work (17). Single-stage and tandem mass spectrometry have been commonly used for quantiï¬cation of drugs in biosamples (17). LC–MS is an excellent completive to GC–MS for quantiï¬cation of thermally labile, high molecular mass, and polar compounds.
Analyte enrichment based on microextraction before CE analysis was proposed for types of drugs of abuse such as amphetamines, phencyclidine, lysergic acid diethylamide, ephedrines, and so on (18–20). In-line preconcentration using a CE capillary inlet to hold a solvent drop to perform SDME is a format worth mentioning. In this setup, a droplet is hanging at the tip of the capillary to perform extraction, and then the droplet is directly injected into the capillary and analyzed (14). For example, a three-phase extraction was proposed to enrich primary-amine compounds, including amphetamine, from urine to the n-octanol layer over the urine sample, and then back-extacted to an acidic droplet suspended at the tip of the CE capillary. The method achieved a 0.5-ng/mL limit of detection (LOD) for amphetamine with ultraviolet (UV) detection (21)
Sample Matrices
Microextraction methods have been developed for analyzing different types of samples, including urine, blood, hair, tissue, and oral fluid. If needed, the sample can be brought into a homogenous aqueous solution by suitable treatment before microextraction.
Urine is often used in forensic toxicology analysis. It can be diluted with buffer or ultrapure water and filtered prior to microextraction. Although the “dilute-and-shoot” approach is used by some laboratories, extraction is still in favour because it prevents instrument contamination and reduces the burden of instrument maintenance.
When analyzing blood (plasma, serum) samples, deproteination is needed before microextraction because proteins may cause significant interference. For example, proteins may be absorbed on the SPME fibre and increase the matrix viscosity, which prevents the efficient extraction of the target analytes. Perchloric acid, trichloroacetic acid, or methanol can used to precipitate proteins (22).
Hair has become increasingly important in determination of substances of abuse in forensic toxicology because it is stable and easy to collect. Compared with specimens such as urine and blood, hair samples provide a wider window of detection (23). Correlated with hair growth rate, the analysis of hair profiles could illustrate the history of drug abuse or poison exposure. Before microextraction, hair samples should be washed to eliminate possible external contamination and then should be cut into small fragments or pulverized (23). Hair samples are often subject to hydrolyzation using a strong base (such as sodium hydroxide) or enzyme to release the target analytes before microextraction (24,25).
Analyzing oral fluid has become popular in recent years, especially when investigating driving under the influence of drugs (4,26). Oral fluid provides information on recent drug use and sample collection is relatively easy, noninvasive, and can be performed under supervision. The sample may have issues with varying viscosity, pH, available amount, and potential external contamination (4). The oral fluid sample is usually diluted or pretreated to remove protein before microextraction. Analytical methods have been developed for determination of amphetamine-type stimulants, cocaine, natural and synthetic opioids and hallucinogens, and antidepressant drugs using SPME or MEPS (4,27).
Application to Forensic Analysis
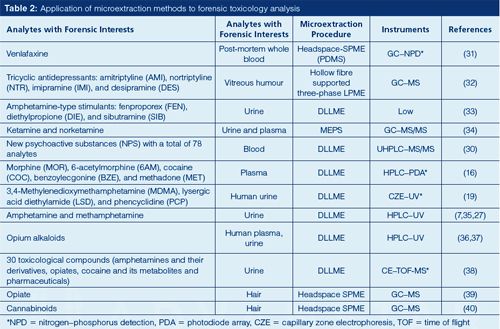
Microextraction methods are typically used for quantification of analytes based on optimum extraction parameters selected for those specific compounds. Several important factors for extraction should be investigated, including type of SPME fibre or type of solvent, sample agitation rate, temperature, pH, and salting effect. The procedure is used for routine analysis after method development and validation. Table 2 lists some examples of using microextraction methods to perform forensic toxicology analysis. It is worth pointing out that, for given analytes, different microextraction approaches could be developed to achieve the desired results. For example, amphetamines (methamphetamine, amphetamine) can be analyzed by using SPME (28), three-phase LPME (7), and headspace single-drop LPME (29).
In addition to quantitative analysis, microextraction shows potential for general purpose screening (22,30). For example, DLLME combined with ultrahigh-pressure liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) was used for the determination of many different classes of drugs of abuse in whole-blood samples (22). Main classes of drugs of abuse (cocaine and metabolites, amphetamines and analogues, lysergic acid, ketamine, opiates including buprenorphine, methadone and fentanyl, and analogues), benzodiazepines, Z-compounds, and other psychotropic drugs were effectively extracted in a single step and determined with satisfactory sensitivity, accuracy, repeatability, and linearity. In this work, chloroform was used as the extraction solvent and methanol was the dispersing reagent. Method LODs varied from 0.05 to 2 ng/L. The developed method was successfully applied to the analysis of 50 blood samples from forensic cases.
Conclusion
Microextraction is a new approach for sample preparation in forensic laboratories. A proper procedure could be developed based on the physicochemical characteristics of target analytes, sample matrix, and available analytical instruments. Analytical problems are often solved by using different microextraction methods and different instruments. Automation and establishing a high-throughput strategy are the development trends in this field, which will help forensic laboratories increase analysis efficiency and reduce the backlog of cases.
References
- R. Jain and R. Singh, Trac-Trends Anal. Chem.75, 227–237 (2016).
- C. Arthur and J. Pawliszyn, Anal. Chem.62, 2145–2148 (1990).
- M. Abdel-Rehim, J. Chromatogr. A1217, 2569–2580 (2010).
- C. Montesano, M.C. Simeoni, R. Curini, M. Sergi, C. Lo Sterzo, and D. Compagnone, Anal. Bioanal. Chem.407, 3647–3658 (2015).
- M.M. Moein, A. Abdel-Rehim, and M. Abdel-Rehim, Trac-Trends Anal. Chem.67, 34–44 (2015).
- Y. He, in Comprehensive Sampling and Sample Preparation, J.L. Pawliszyn, Ed. (Academic Press, Oxford, UK, 2012), Vol. 3, pp. 835–862.
- Y. He and Y.-J. Kang, J. Chromatogr. A1133, 35–40 (2006).
- M. Rezaee, Y. Assadi, M.R.M. Hosseinia, E. Aghaee, F. Ahmadi, and S. Berijani, J. Chromatogr. A 1116, 1–9 (2006).
- M. Mohamadi and A. Mostafavi, Talanta 81, 309–313 (2010).
- J. Cheng, Y.T. Xia, Y.W. Zhou, F. Guo, and G. Chen, Anal. Chim. Acta701, 86–91 (2011).
- X.L. Song, J.H. Li, C.Y. Liao, and L.X. Chen, Chromatographia74, 89–98 (2011).
- X.W. You, Z.K. Xing, F.M. Liu, and N.W. Jiang, J. Chromatogr. A1311, 41–47 (2013).
- R.S. Zhao, X. Wang, J. Sun, C. Hu, and X. K. Wang, Microchim. Acta174, 145–151 (2011).
- H.Y. Xie, Y.Z. He, W.E. Gan, C.Z. Yu, F. Han, and D.S. Ling, J. Chromatogr. A1217, 1203–1207 (2010).
- T.N. Posch, M. Putz, N. Martin, and C. Huhn, Anal. Bioanal. Chem.407, 23–58 (2015).
- P. Fernandez, M. Regenjo, A.M. Bermejo, A.M. Fernandez,
- R.A. Lorenzo, and A.M. Carro, J. Appl. Toxicol. 35, 418–425 (2015).
- H.H. Maurer, Anal. Bioanal. Chem.381, 110–118 (2005).
- T. Baciu, I. Botello, F. Borrull, M. Calull, and C. Aguilar, TracâTrends Anal. Chem.74, 89–108 (2015).
- D. Airado-Rodriguez, C. Cruces-Blanco, and A.M. GarciaâCampana, J. Chromatogr. A1267, 189–197 (2012).
- U. Alshana, N.G. Goger, and N. Ertas, J. Sep. Sci. 35, 2114–2121 (2012).
- K. Choi, J. Kim, Y.O. Jang, and D.S. Chung, Electrophoresis30, 2905–2911 (2009).
- M. Fisichella, S. Odoardi, and S. Strano-Rossi, Microchem. J.123, 33–41 (2015).
- S. Vogliardi, M. Tucci, G. Stocchero, S.D. Ferrara, and D. Favretto, Anal. Chim. Acta857, 1–27 (2015).
- F.L. Roveri, B. Paranhos, and M. Yonamine, Forensic Sci. Int.265, 75–80 (2016).
- M. Miguez-Framil, A. Moreda-Pineiro, P. Bermejo-Barrera, P. Lopez, M.J. Tabernero, and A.M. Bermejo, Anal. Chem.79, 8564–8570 (2007).
- D.Z. Souza, P.O. Boehl, E. Comiran, K.C. Mariotti, F. Pechansky, P. Duarte, R. De Boni, P.E. Froehlich, and R.P. Limberger, Anal. Chim. Acta696, 67–76 (2011).
- M. Wozniakiewicz, R. Wietecha-Posluszny, A. Moos, M. Wieczorek, P. Knihnicki, and P. Koscielniak, J. Chromatogr. A1337, 9–16 (2014).
- Y. He, J. Pohl, R. Engel, L. Rothman, and M. Thomas, J. Chromatogr. A1216, 4824–4830 (2009).
- Y. He, A. Vargas, and Y.-J. Kang, Anal. Chim. Acta589, 225–230 (2007).
- S. Odoardi, M. Fisichella, F.S. Romolo, and S. Strano-Rossi, J. Chromatogr. B1000, 57–68 (2015).
- O. Mastrogianni, G. Theodoridis, K. Spagou, D. Violante, T. Henriques, A. Pouliopoulos, K. Psaroulis, H. Tsoukali, and N. Raikos, Forensic Sci. Int.215, 105–109 (2012).
- M.F. dos Santos, A. Yamada, S.C. Seulin, V. Leyton, C.A.G. Pasqualucci, D.R. Munoz, and M. Yonamine, J. Anal. Toxicol.40, 187–193 (2016).
- R.L. Cunha, W.A. Lopes, and P.A.P. Pereira, Microchem. J. 125, 230–235 (2016).
- I. Moreno, M. Barroso, A. Martinho, A. Cruz, and E. Gallardo, J. Chromatogr. B1004, 67–78 (2015).
- T. Ahmadi-Jouibari, N. Fattahi, and M. Shamsipur, J. Pharm. Biomed. Anal. 94, 145–151 (2014).
- T. Ahmadi-Jouibari, N. Fattahi, M. Shamsipur, and M. Pirsaheb, J. Pharm. Biomed. Anal.85, 14–20 (2013).
- M. Shamsipur and N. Fattahi, J. Chromatogr. B879, 2978–2983 (2011).
- I. Kohler, J. Schappler, T. Sierro, and S. Rudaz, J. Pharm. Biomed. Anal.73, 82–89 (2013).
- M. Moller, K. Aleksa, P. Walasek, T. Karaskov, and G. Koren, Forensic Sci. Int. 196, 64–69 (2010).
- C.D.R. de Oliveira, M. Yonamine, and R.L.D. Moreau, J. Sep. Sci. 30, 128–134 (2007).
Yi He is a professor of chemistry at John Jay College of Criminal Justice, the City University of New York, USA. Her research interests include development of novel sample preparation techniques, microextraction in specific, and their applications to forensic and environmental analysis; elucidation of multielement fingerprints of forensically important trace evidence; and investigation of trace toxic elements (arsenic, cadmium, and lead) in samples with forensic and public health interests. She is also active in developing effective learning models to promote science education.
“Sample Prep Perspectives” editor Douglas E. Raynie is an associate research professor at South Dakota State University, USA. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his Ph.D. in 1990 at Brigham Young University under the direction of Milton L. Lee.

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.












