Method Selection for the Determination of Suspected Allergens in Essential Oils, Flavour, Fragrances and Cosmetics
LCGC Europe
When possible, GC analysis is performed under retention time locked conditions, meaning that allergen elucidation and quantification is based on mass spectral data (scan mode or specific ion detection mode) and on retention time.
According to EU Directive 2003/15/EC, 24 compounds listed as suspected allergens have to be determined qualitatively and quantitatively in fragranced products because they may cause skin irritation.1 The list, presented in Table 1, is mainly composed of natural products and some of the solutes consist of more than one chemical identity. Citral possesses two isomers namely neral (Z citral) and geranial (E citral); Lyral also contains two isomers named Lyral 1 and Lyral 2, while farnesol consists of at least four possible isomers with the Z,E (farnesol 1) and E,E isomer (farnesol 2) are predominant. The presence of the listed allergens in cosmetic products should be labelled when their concentration exceeds 0.001% (10 ppm) in leave-on products and 0.01% (100 ppm) in rinse-off products. In addition to the list, some related compounds such as phenylacetaldehyde, estragole, methyl-2-nonynoate and methyleugenol are also currently monitored.2 Method development should therefore cover 31 target compounds in total.

Table 1: List of suspected allergens with respective CAS numbers.
The range of matrices in which the target compounds have to be measured is very broad and includes natural essential oils, synthetic mixtures of flavour and fragrance compounds, natural product extracts and finished products such as soaps, gels, shower gels, lipstick and other cosmetic products. Moreover, the range of concentrations of the fragrance compounds in these matrices is very broad (from ppm to %). It is clearly impossible to determine all target compounds in all different matrices using one single method.
Therefore, we have proposed to classify the matrices in four classes (Table 2).3 For each class, dedicated analytical methods have been developed and validated.

Table 2: Classification of samples for the analysis of suspected allergens based on matrix characteristics.
Class I is composed of samples that contain volatile or semi-volatile solutes typically eluting on an apolar capillary column between n-decane (retention index 1000) and n-docosane (retention index 2200). The complexity of the sample and the concentration range in which the solutes are present is not very high and non-volatile matrix compounds are not present.
Typical examples are essential oils (obtained by distillation) or synthetic fragrance mixtures containing up to approximately 100 solutes. For this class of samples, direct injection of a diluted sample in combination with gas chromatography–mass spectrometry (GC–MS) either in scan4 or in selected ion monitoring (SIM) mode is used.2 Class II also consists of samples only containing volatile and semi-volatile solutes, but their complexity is high (> 100 solutes) and/or the concentration range is very broad (for example, very low concentrations of target compounds next to very high concentrations of matrix compounds). For these complex samples, a single dimension gas chromatographic separation might be insufficient. The capabilities of two-dimensional capillary GC (GC–GC) and comprehensive GC (GC×GC) have been evaluated by several groups for this application.5–8 A comparison between GC–GC and GC×GC for a typical perfume sample is presented further.
In contrast to classes I and II, samples in class III contain non-volatile material (solutes eluting after n-hexacosane). A typical example of a class III sample is an extract from plant material such as Mimosa absolute oil. These samples contain high molecular weight material such as waxes, sterols, fatty acids, fatty alcohols, flavanoids etc. Direct injection of these samples as a solution or an extract is no longer possible because the non-volatile material will contaminate the inlet and column after a few injections. For this sample class, a programmed temperature vapourizing (PTV) inlet with automated liner exchange (ALEX) was used. In this technique fractionation of the target compounds from the non-volatile matrix is taking place in a special liner packed with polydimethylsiloxane (PDMS) during injection.9 Finally, class IV contains finished products (lotions, shampoo, cosmetic products in general). In these samples the target solutes are normally present at relatively low concentrations, whereas the matrix can be quite complex because of the presence of glycols, non-ionic and ionic surfactants, for example. For class IV samples, the automated liner exchange-PTV approach can be used9 but, in addition, aqueous-based samples can be analysed using stir bar sorptive extraction (SBSE). This solventless technique was already successfully applied to many different aqueous matrices, both for quality control (QC) and for trace analysis.10
An overview is presented of the different analytical methods and the performances are illustrated with the determination of suspected allergens in different types of matrices. The chromatographic approach is always based on GC–MS using a benchtop single quadrupole system operated in scan, SIM or simultaneous scan/SIM mode. When possible, GC analysis is performed under retention time locked conditions, meaning that allergen elucidation and quantification is based on mass spectral data (scan mode or specific ion detection mode) and on retention time (Table 2).
Experimental Details
Solutes and solutions: The target solutes were obtained as p.a. grade chemicals (Sigma-Aldrich, Bornem, Belgium) or obtained as a gift from Robertet (Grasse, France) or Firmenich (Geneva, Switzerland). Individual stock solutions at 10 mg/mL level were prepared in iso-octane or acetone (pesticide grade, Merck ChromaSolv, VWR, Leuven, Belgium). From the individual stock solutions, calibration mixtures were prepared by dilution in dichloromethane or acetone (depending on the analytical method). To the calibration solutions and samples, 1,4-dibromobenzene (IS1) and 4,4'-dibromobiphenyl (IS2) were added as internal standards (Sigma-Aldrich).
Instrumental conditions for class I samples (direct injection — GC–MS): For the analysis of class I samples a 5% (m/v) dilution of the sample in dichloromethane or acetone is used. To the sample solution, both internal standards are added in 100 ng/μL concentration to correspond with 0.2% (w/w) in the sample.
A 6890 GC (Agilent Technologies, Little Falls, Delaware, USA) was used in combination with a 5973 inert or a 5975 mass spectrometric detector (Agilent Technologies). Split injection (1 μL) was performed using a split-splitless injector at 250 °C and a split ratio of 1/25. The separation was done on a 30 m × 250 μm i.d., 0.25 μm df HP–5MS column (Agilent Technologies). The oven was programmed from 50 °C (1 min) to 270 °C at a rate of 8 °C/min (total analysis time 28.5 min). Helium was used as carrier gas at a constant pressure of approximately 70 kPa (1.2 mL/min at 50 °C). The pressure was adjusted to elute alpha-isomethyl ionone at 15.5 min using the retention time locking option on the 6890 GC.
The 5973 MSD was used in the scan mode (40–350 amu) or in the SIM mode. Recently, the method was transferred to a 5975 MSD operated in simultaneous scan/SIM mode. The SIM programme (on both instruments) was generated using the AUTOSIM option. For each solute, three ions were selected (one ion is removed from the automatic selection of the 4 most abundant ions) and these m/z values were transferred to the data acquisition programme. Most of the selected ions correspond to the ions proposed by Chaintreau et al. in the reference method.2 Target compounds, the locked retention times, the target ion (quantification ion), the qualifiers and the SIM groups are listed in Table 3. Calibration curves were prepared using 1 to 100 ppm solutions. The injected amounts correspond to 40 to 4000 pg per compound (1/25 split ratio) or to 20 to 2000 μg/g sample (for a 5% diluted sample). Extra dilution can be necessary, depending on the samples, since some essential oils contain very high concentrations (> 1%) of some target compounds (Table 3).
Table 3: List of target compounds, with the corresponding locked retention times, SIM groups, target ions (quantification ion), and qualifier ions (Qual). Quantitative data obtained by simultaneous scan/SIM acquisition are included, linearity (1â100 ppm range) and LOD (mg/kg sample corresponding to S/N 5 3) for scan and SIM mode respectively.
Instrumental conditions for class II samples (multidimensional GC): For the analysis of class II samples a 5% dilution of the sample in dichloromethane or acetone is used. The samples are injected directly without additional clean-up. Two analytical configurations were tested.
First experiments were made on a 6890 GC equipped with FID detection. The system was equipped with a KT2004 thermal modulator (Zoex Corporation, Houston, Texas, USA).11 A 30 m × 250 μm i.d., 0.25 μm df HP-5MS column was used as the first dimension and a 2 m × 100 μm i.d., 0.25 μm df DB-WAX column (Agilent Technologies) was used as the second dimension. The thermal modulator consisted of a loop type interface of 1 m × 100 μm i.d. deactivated fused silica. The modulation time was set at 6 s. Injection (1 μL) was done on a split/splitless inlet operated in split mode (1/100 split ratio). The oven temperature was identical to the programme described above (50 °C – 1 min – 8 °C/min – 270 °C). Helium was used at 200 kPa constant pressure. No attempts were made to lock the retention times and, therefore, the elution times of the target compounds deviate from the retention times obtained with method 1. FID detection was performed at 100 Hz data acquisition rate and data analysis was done using GC Image software (Zoex).
Secondly, a two-dimensional multi-column switching (MCS) system (Gerstel GmbH, Mülheim an der Ruhr, Germany) was used. The system consisted of two 6890 GCs coupled via a heated transfer line (CTS, Gerstel). Injection (1 μL, 1/25 split ratio, 250 °C) was done on a split/splitless inlet located on the first GC. A 30 m × 250 μm i.d., 0.25 μm df HP-5MS column was used as the first dimension column. The outlet of the column is coupled to a multi-column switching device.12 The separation in the first dimension was monitored using FID detection (first GC). Selected fractions are heart-cut onto a cold trap (CTS, Gerstel) and injected (-50 °C to 250 °C at 10 °C/s after the end of the last heart-cut) into a second dimension column. A 30 m × 250 μm i.d. 0.25 μm df DB-WAX column was used as second dimension column. The initial oven temperature of the second dimension was kept at 50 °C until all fractions were heart-cut and injected. The DB-WAX column was then programmed at 8 °C/min to 250 °C. The head pressure of the second dimension column was set at 60 kPa. The inlet pressure (on the first dimension column) was adjusted to elute alpha-isomethyl ionone at 15.5 min (approximately 215 kPa). Detection was by MS in scan mode (40–350 amu).
Instrumental conditions for class III samples (ALEX — PTV — GC–MS): For class III samples, sample preparation is done by extraction/solubilization of 1 g sample in 10 mL dichloromethane. Both internal standards are added to a concentration of 5 μg/mL, corresponding to 50 ppm in the sample. Extraction/solubilization is performed by ultrasonic agitation during 15 min. From the centrifuged extract, an aliquot is transferred to an autosampler vial and analysis is performed on a 6890 GC coupled to a 5973 inert mass spectrometric detector and equipped with a CIS–4 PTV injector (Gerstel). Automated injection was performed using a Gerstel multipurpose MPS–2 sampler equipped with an automatic liner exchange device (ALEX–Gerstel). The PTV–ALEX combination allows standard liquid injection and automated replacement of liners. Compared with a classical PTV inlet, the septum cap or septumless head of the PTV is replaced by a special device that automatically opens and closes before and after introduction of a new liner. Special liners (2 mm i.d., packed with a 1 cm bed of PDMS foam) were developed to retain the non-volatiles in the liner.9 The liners are inserted in an ALEX transport adapter equipped with a 5 mm diameter × 3 mm thick septum, through which liquid injection is made. The PDMS packed liners are available from RIC and Gerstel GmbH. The same analytical conditions as described above are applied (column, temperature programme, retention time locking). The PTV conditions were 1 μL injection, split 1/25, PTV temperature programme 0 °C – 0.05 min – 12 °C/s – 250 °C – 2 min. Calibration was done using diluted standard solutions with concentrations in the range from 0.5 to 20 μg/mL.
Instrumental conditions for class IV samples (SBSE — TDU–GC–MS): An Agilent 6890 GC, equipped with a CIS-4 inlet and coupled to an Agilent 5975MSD was used. An aliquot of the sample (typically 100–250 mg) is diluted in 5 mL of a 1:9 ethanol/water mixture (20 to 50-fold dilution). One microlitre of both internal standards (1% solution) are added corresponding to 100 ppm (μg/g sample). Calibration is done by spiking the solutes also in 5 mL of a 1:9 ethanol/water mixture (concentrations between 20 and 1000 μg/mL corresponding to 1–50 ppm in the sample for a 100 mg/10 mL sample). SBSE extraction was done using a 10 mm × 0.5 mm PDMS df stir bar at room temperature during 1 h while stirring at 900 rpm. After extraction, the stir bar is shortly rinsed in 5 mL water, dried on a tissue paper and introduced in a thermal desorption unit (TDU) (Gerstel) installed on top of the PTV inlet. The analytical conditions are similar to the conditions described above. The TDU was programmed from 30 °C to 250 °C at 60 °C/min. The desorption flow was 50 mL/min and desorption was done in splitless mode. The desorbed compounds were trapped in an empty liner at –100 °C. After desorption, the PTV was programmed to 250 °C at 10 °C/s and injection was done in split (1/10) mode. The MSD was operated in scan mode (40–350 amu).
Results and Discussion
Analysis of class I samples by direct GC–MS analysis using retention time locked GC conditions and simultaneous scan/SIM mode: The analysis of suspected allergens can be performed on several columns. In a proposed reference method,2 a 100% dimethylsiloxane column (HP–1, DB-1) or a 50% phenylmethyl siloxane (DB–17) have been selected because of their good thermal stability and low bleeding. An HP–1 column was also used for the analysis of allergens in non-volatile matrices.9 We have experienced that equally good results can be obtained in a 30 min analysis time on a 30 m × 0.25 mm i.d. × 0.25 μm df HP–5MS column (5% phenyl methylsiloxane). Because this column is often considered as the standard column for GC–MS work, it was used throughout this work. The separation of a mixture of the 31 individual solutes plus 2 internal standards on the HP-5MS column is shown in Figure 1. Most solutes are well separated within 25 min and the critical pairs can easily be deconvoluted using extracted ions. Other columns may result in better resolution of some solute pairs, but in real QA/QC work, the final applicability of the column is determined by the complexity of the matrix and possible co-elution of target solutes with matrix compounds that are often quite similar in chemical structure (terpenes, terpenoids). For the analysis of suspected allergens in real perfume samples, fast capillary GC using narrow bore columns was also evaluated. Analysis times can be reduced to less than 10 min for essential oil analysis, but for samples with relatively large concentration differences, problems were observed because of the limited sample capacity of narrow bore columns. Therefore, all described methods are based on standard bore (0.25 mm i.d.) columns.
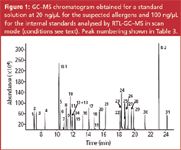
Figure 1
The method described in the experimental section was retention time locked (RTL) using alpha-isomethyl ionone as the locking standard. A mass spectral library was created containing the 27 suspected allergens, the 4 extra solutes and the 2 internal standards. The retention times were included in the mass spectral library and a screener library was created. This library can be used to screen samples for the presence of the target solutes, based on mass spectral information and on retention time data. The retention times under RTL conditions are given in Table 3. The allergen mass spectral library was also transformed into an automated mass spectral deconvolution and identification system (AMDIS) target compound library. Peak identification is thus also possible using deconvoluted spectra. The obtained results from the Agilent Chemstation quantification report can be combined with the results from mass spectral identification in a deconvolution reporting software (DRS) report.13
A typical set of chromatograms obtained for the analysis of a perfume sample using the simultaneous scan/SIM mode on an Agilent 5975 MSD is shown in Figure 2. The upper chromatogram shows the scan trace. The lower chromatogram shows the SIM trace. At approximately 7–8 min in the scan chromatogram, a broad set of peaks is detected. These peaks correspond to propyleneglycols, used as keeper solvents for fragrances. In the same elution window limonene and benzyl alcohol elute. These compounds can easily be detected in the SIM trace (peaks 1 and 2). Other suspected allergens could also be detected. Since both chromatograms are available, the SIM data can be used for selective detection and quantification. The scan trace can be used for confirmation of peak identity. During these tests it has been observed that the linearity and the sensitivity obtained by using the simultaneous scan/SIM mode were very similar compared with separate scan or SIM acquisition, respectively.
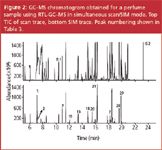
Figure 2
To check quantitative performance, a series of calibration solutions were analysed using scan/SIM mode. For each target solute, the linearity and limit of detection (LOD), corresponding to the amount that can be detected with a signal-to-noise (S/N) ratio of 3, was determined. The target solutes eluting before 17 min are calibrated using IS1 (dibromobenzene), the allergens eluting after 17 min are calibrated versus IS2 (dibromobiphenyl). The results obtained from the scan and SIM traces, respectively, are included in Table 3. For all solutes, the linearity was better than 0.99 both in scan and in SIM mode. The limit of detection in scan mode is between 0.2 and 3 ng injected, corresponding to 6 to 60 ppm in the matrix. The limit of detection obtained in SIM mode is 5 to 100 pg, corresponding to 0.1 to 2 ppm in the matrix. These data are obtained using a 5% diluted sample, 1 μL injection and a 1/25 split ratio.
Analysis of class II samples by two-dimensional capillary GC (GC–GC) and comprehensive GC (GC×GC): When analysing perfume samples, problems can be encountered because of the complexity of the sample. For example, during the analysis of the perfume sample (Figure 2) the presence of some target compounds could not be confirmed. In the elution window between 18 and 19 min (indicated with a question mark in Figure 2), the Lyral isomers could not be unequivocally elucidated. These compounds were probably present but their spectra didn't match the library spectra (scan) or the relative ion abundances were out of range (SIM). Therefore, both GC×GC and GC–GC were tested on this sample.
Several groups have demonstrated that GC×GC offers very high peak capacities and very detailed profiles of essential oil mixtures can be obtained.5–8 The technique is very well suited to compare different batches of complex essential oils. However, contrary to several applications such as petroleum product analysis,14 essential oils and flavour and fragrance mixtures do not show "ordering" in the chromatograms making the obtained plots difficult to interpret. The lack of ordering is a result of the very broad range of polarities and volatilities, which results in scattered plots. This is illustrated in Figure 3, showing the separation of a standard mixture containing 139 flavour and fragrance solutes, including all suspected allergens, and a series of n-alkanes. Most solutes are well resolved. The n-alkanes can easily be detected in the lower part of the plot as they elute fast on the second dimension polar column. The other solutes are "scattered" and no group-type separation is obtained.

Figure 3
The contour plot of the comprehensive GC×GC–FID separation of the same perfume sample as in Figure 2 is shown in Figure 4. Some target solutes could easily be detected by comparing the first and second dimension retention times with the retention times obtained for the standard solutes under identical conditions. In this way, for instance, coumarin could be detected at approximately 18.5 min (1st dimension) and 5.5 s (2nd dimension). No mass spectral confirmation was done and the retention times were not locked using the GC×GC configuration used.

Figure 4
For detection of other target compounds, however, a major problem was observed in the GC×GC separation because of the limited sample capacity of the second dimension column. Since only a short length of narrow bore column is used, a very limited sample capacity is available.15 The injected amount was already reduced (split 1/100 instead of 1/25), but still severe overloading is observed. This is illustrated through the very large bands obtained for propyleneglycol (around 9 min in the first dimension). Under these conditions, benzyl alcohol could not be detected. Also for the complex fraction at 19–20 min (Lyral isomers), insufficient resolution was obtained on the second dimension separation. The lack of chromatographic "ordered" chromatograms and the limited sample capacity in the second dimension thus lead to a more difficult detection of trace compounds in perfume samples. Other studies such as described in reference 5 came to the same conclusion. The use of mass spectrometric detection is thus mandatory. The group of A. Chaintreau et al. has reviewed the use of a single quadrupole mass spectrometer for the quantification of suspected allergens.6,16 For comprehensive GC×GC, a fast scanning time-of-flight MS (TOF–MS) is by far the best choice but it is questionable whether such expensive and specialized instrumentation, is economically realistic in a QA/QC environment.
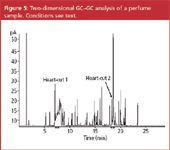
Figure 5
The same perfume sample was analysed on a classical two-dimensional system. The separation obtained on the first dimension is shown in Figure 5. Two heart-cuts at respectively 7–8 min and 18–19 min were performed in the same run. The separation obtained on the second dimension column is shown in Figure 6.
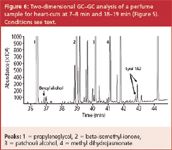
Figure 6
Limonene elutes at 14.06 min (not shown) and can easily be detected and quantified. Now also benzylalcohol (36.87 min) and both Lyral isomers (42.79 and 42.90 min) could be detected and quantified, although high abundant matrix compounds are present in the same elution window from the first dimension separation. Since a standard column is used in the second dimension, the sample capacity is higher and good peak shapes are obtained for the highly abundant peaks, such as propyleneglycol (Figure 6, peak 1, 36.45 min).
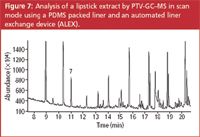
Figure 7
Analysis of class III sample by ALEX-PTV — GC–MS: The major problem with the analysis of class III and class IV samples is the presence of high molecular weight compounds that contaminate the analytical system (inlet and column) and quickly damage its performance. Therefore, a programmed temperature vapourization (PTV) inlet was used. Although PTV injection allows a controlled temperature programming of the inlet, fractionation of the sample by temperature only (distillation) is not selective enough to allow quantitative introduction of the volatile analytes, on one hand, and sufficient retention of the high molecular weight components, on the other hand. To increase the fractionation capabilities, packing material in the liner is required. Best results in terms of inertness and sample capacity were obtained using a 2 mm i.d. PTV liner packed with a 1 cm bed of PDMS foam.9 A typical application of this method is the analysis of lipstick. A lipstick extract in dichloromethane was injected on the PDMS packed liner and analysed using the conditions described in the experimental part. The resulting chromatogram is shown in Figure 7. The presence of citronellol could easily be detected at 10.99 min and confirmed by its the mass spectrum. Although some hydrocarbons and waxes are detected at the end of the chromatogram, most of the high molecular weight matrix compounds remain in the liner, which is removed after analysis, while the analytical system (column) is not contaminated. The same method was successfully applied to the analysis of natural product extracts containing oleoresins (Mimosa absolute oil) and to some class IV samples, such as detergent powder and shampoo.9
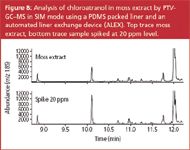
Figure 8
Natural extracts also include two lichen extracts (tree moss and oak moss) that are also mentioned in the EU directive.1 The moss extracts can be analysed using the ALEX–PTV method. Figure 8 shows the determination of chloroatranol (2,6-dihydroxy-3-chloro-4-methyl-benzaldehyde) in a moss extract. A 5% dilution of the moss extract in dichloromethane was injected on the PDMS liner. Detection was done in MS-SIM mode, monitoring ions m/z 185 and 187 for chloroatranol and m/z 199 and 201 for the internal standard 2,6-dihydroxy-3-chloro-4-ethyl-benzaldehyde. The upper trace (extracted ion m/z 185) is obtained for the sample. Chloroatranol is detected at 10.1 min. The lower trace is obtained for the same sample spiked with 20 ppm chloroatranol. Since the peak area is approximately doubled, the concentration of chloroatranol in the non-spiked sample is in the order of 20 ppm (20 μg/g sample). The chromatograms are very clean and detection of the target compound can be done without interference. After removal of the liner, a green colour caused by retained chlorophyll could be observed on the PDMS packing indicating that removal of non-volatile material was successful.
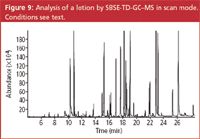
Figure 9
Analysis of class IV samples by stir bar sorptive extraction — thermal desorption — GC–MS: For the analysis of class IV samples (finished products), the same method as described above can be used. In some instances, the concentration of the fragrance products is, however, quite low while also the level above which labelling is mandatory, is low (10 ppm for "leave-on" products).1 Stir bar sorptive extraction (SBSE) was evaluated as an alternative method. SBSE is a solventless extraction method that allows the extraction of compounds from an aqueous sample into a polydimethylsiloxane (PDMS) phase.17 The extraction efficiency is dependent on the phase ratio (sample volume/volume PDMS) and on the octanol–water partitioning coefficient. For most of the allergens, the Ko/w is sufficiently high to provide high extraction efficiencies. The most critical compound is benzylalcohol (log Ko/w = 1.1), but even this compound can be measured by SBSE using a relatively small (5 mL) sample volume.
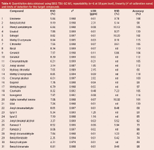
Table 4: Quantitative data obtained using SBSE-TDU-GC-MS, repeatability (n56 at 50 ppm level), linearity (r2 of calibration curve) and limits of detection for the target compounds.
The linearity and limit of detection were determined by the analysis of 8 solutions of target compounds in an ethanol-water (10/90) mixture at concentrations between 1 and 50 ppm (μg/g sample). The addition of ethanol prevents wall adsorption and also simulates samples containing alcohol. The repeatability (n = 6 at 50 ppm level), linearity (r2 of calibration curve) and limits of detection for the target compounds are listed in Table 4. The limit of detection (LOD), expressed in ppm or μg/g sample, is calculated as the amount that can be detected with S/N = 3. The value was calculated from the lowest calibration point. In general, the relative standard deviations (RSDs) are in the order of 5–10% and the LODs are below 1 ppm for most solutes. For the alcohols (benzyl alcohol, anisyl alcohol, hydroxy citronellal and cinnamyl alcohol), the LOD is around 2 ppm using the MS in scan mode. The repeatability was tested for a lotion sample. The chromatogram of the sample is given in Figure 9. A zoom of the elution window between 19 and 24 min is given in Figure 10. At 19.58 min, hexyl cinnamaldehyde was detected. The analysis of this sample was repeated 6 times and the RSDs on the measured concentrations are included in Table 4. To the same sample, 50 ppm of each target compound was added and the accuracy was determined (measured concentration versus added concentration, in %). These values are also included in Table 4. The accuracy for most solutes is between 70% and 120%, while the RSDs are in the order of 5–10%.
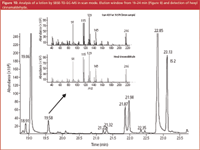
Figure 10
The analysis of a shampoo sample by SBSE and operating the MS in the scan mode is shown in Figure 11. The insert corresponds to the same sample spiked with 2 ppm amyl cinnamyl alcohol clearly illustrating the sensitivity of the method.
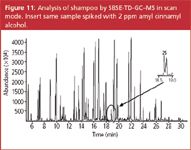
Figure 11
Conclusions
Retention time locked GC–MS methods are presented for the qualitative and quantitative analysis of allergens in fragranced samples. For class I samples, direct injection GC–MS operated in scan/SIM mode can be used. For complex samples, comprehensive GC×GC can be used to obtain a total sample pattern, but for the analysis of trace solutes selective detection (fast scanning MS) is required. Alternatively, classical two-dimensional GC using heart-cutting can be used. The higher sample capacity of the second dimension column allows better separation of target compounds from abundant matrix compounds. Class III and IV samples, containing non-volatile matrix compounds are analysed using PTV injection on a PDMS packed liner and automated liner exchange. Finally, for diluted samples, stir bar sorptive extraction, followed by thermal desorption GC–MS is a good alternative for method 3. Good linearity, repeatability and sufficient sensitivity are obtained by the different methods. By using retention time locking and automated deconvolution software, identification of target solutes is straightforward.
Acknowledgements
Dr A. Chaintreau (Firmenich SA, Geneva, Switzerland) and Dr D. Joulain (Robertet SA, Grasse, France) are thanked for supplying the standards and for discussion of this project.
Frank David is R&D manager at the Research Institute for Chromatography (RIC).
Christophe Devos is research scientist at RIC. His research focuses on the automation of GC analyses.
Pat Sandra is director of RIC and professor at the Ghent University, Belgium.
References
1. Directive 2003/15/EC, Official Journal of the European Union, L 66/26, 11.3.2003.
2. A. Chaintreau et al., J. Agric. Food Chem., 51, 6398–6403 (2003).
3. P. Sandra and F. David, papers presented at the 35th International Symposium on Essential Oils (ISEO), Sicily, 29 September–2 October 2004, at the International Symposium on Chromatography, Paris, 4–8 October 2004 and at the 36th ISEO, 4–7 September 2005, Budapest.
4. H. Leijs et al., J. Agric. Food Chem., 53, 5487–5491 (2005).
5. R. Shellie, P. Marriott and A. Chaintreau, J. Flavour Fragr., 19, 91–98, (2004).
6. C. Debonneville and A. Chaintreau, J. Chromatogr. A, 1027, 109–112, (2004).
7. L. Mondello et al., J. Chromatogr. A, 1067, 235–243, (2005).
8. M. Adahchour et al., J. Chromatogr. A, 1067, 245–254, (2005).
9. F. David et al., J. Sep. Science, 29, 1587–1594 (2006).
10. F. David, B. Tienpont and P. Sandra, LCGC N. Am., 21, 108–118 (2003).
11. E.B. Ledford, J.R. TerMaat and C.A.Billesbach, Technical Note KT 030606-1, www.zoex.com.
12. K. MacNamara, R. Leardi and A. Hoffmann, LCGC Eur., 12a, 14–22, (2003).
13. Ph.L. Wylie et al., Agilent Technologies application note 5989-1157EN (2004).
14. R. Edam et al., J. Chromatogr. A, 1086, 12–20, (2005).
15. L.M. Blumberg, J. Chromatogr. A, 985, 29–38, (2003).
16. C. Debonneville, M.A. Thome and A. Chaintreau, J. Chromatogr. Sci., 42(8), 450–455, (2004).
17. E. Baltussen et al., J. Microcolumn Sep., 11, 737–747, (1999).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









