Method or System?
Where to place the blame when something goes wrong.
Many of us use liquid chromatography (LC) methods supplied to us by others. These can be compendial methods — those that come from one of the pharmacopoeia — or they can come from the scientific literature or from another laboratory within our company. Such methods can be very specific in terms of the column and mobile phase to be used or they might be more general, so that the end user has some flexibility in adapting the method to his or her needs. One of the most common traits of such methods, however, is that the person who developed the method is not available to field our questions about the method. And the method does not usually have any background on why certain conditions were chosen. This month's "LC Troubleshooting" discusses the type of problems one can encounter when using such methods.
The method in question specified a 150 mm × 4.6 mm, 5 µm particle C18 column (brand not listed) and a mobile phase of 57% methanol and 43% 10 mM ammonium carbonate buffer, pH 9.6, run at 1.5 mL/min. The column temperature was not specified. The sample is prepared by dissolving a drug tablet in water, filtering it and injecting 20 µL. The separation is simple, and with typical retention times of 2.5 and 3.7 min for the two components of interest, the requirement of resolution, Rs, of more than 2 is met easily. However, the required tailing factor, TF, of ≤1.5 is more of a challenge. Typically, for the second peak, TF = 1.4–1.45, but for the first peak, TF = 1.4–1.5 with a new column, but degrades to TF > 1.5 after 1000–1500 injections. Installation of a new column solves the problem. I was asked how to fix this method.
Method or System?
One of the first questions that I ask when presented with a problem method is whether the problem really relates to the method itself or if it is a problem with the LC system hardware. We can have the best method in the world, but if there is a problem with the LC system, we might not be able to use it. Fortunately, there is a simple way to check the system for reasonable performance. Just replace the column with a new C18 column and repeat part of the column manufacturer's column test. This can usually be accomplished by equilibrating the column in the mobile phase that was used to test the column, often 60–70% methanol–water run at a flow-rate of 1 mL/min. If you have the test sample, use it. Otherwise, nearly any well-retained neutral aromatic compound will work — toluene, methyl benzoate, naphthalene and so forth. The mobile phase should generate a peak that is retained with a retention factor, k, of at least 2. The retention factor is calculated, as
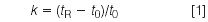
where tR is the retention time of the compound and t0 is the column dead-time (solvent front), normally determined by injecting an unretained compound, such as uracil or thiourea. You should observe a column plate number (also known as column efficiency) that is within 5–10% of the manufacturer's value and a peak that is nearly symmetrical (0.9 < TF < 1.2).
Another way to be sure the system is working properly is to check its performance with a well-behaved method for another sample that you use regularly. Once you have checked out the system and it is determined to be OK, then you can point your finger at the method as the problem. Otherwise, you should correct any system problems before proceeding. In the present example, I recommended running a system check. For the remainder of this discussion, I will assume that it passed.
What Could Be Wrong?
After I am convinced that the LC system is working properly, I look through the method variables and list each item that might be causing the problem. Let us look at the possibilities:
Column size and flow-rate: The 150 mm × 4.6 mm, 5 µm particle diameter column is the workhorse of the LC industry and is about as trouble-free as a column can be. These columns are quite tolerant of pressure, sample size, flow-rate and other variables. When narrower diameter or shorter columns are used, extracolumn peak broadening can be an issue, so more care must be taken of the system plumbing and injection process. Smaller particles, while generating higher plate numbers, create more pressure and require smaller frits, which are blocked more easily. The 150 mm × 4.6 mm, 5 µm column is used widely at 1–2 mL/min flow-rates without generating excess pressure. So I doubt that the problem is related directly to the column size or flow-rate.
Column type: The C18 column is the most widely used column packing material for LC separations. However, the specific column brand and model were not identified. This is often the case for compendial methods, but less so for methods from the literature or for methods developed in-house. At one time we thought that all C18 columns were equivalent, but that assumption was discarded many years ago. For the present method, two major considerations come to mind. First, nearly all "modern" LC columns — those developed in the last 15 years or so — are based upon high-purity silica, often called Type B silica. These are more stable, less variable from column to column and less likely to have tailing problems than the older, less pure Type A silica columns. The distinction between Type A and B is not black and white, but is a continuum, generally (but not absolutely) with the newer Type B columns being of higher purity than the older Type B ones.
Another characteristic of the higher purity columns is their stability at high pH. As a general rule, silica-based C18 columns can be used in the 2 < pH < 8 range without problems. Drop the pH below 2 and the bonded phase tends to hydrolyse. Above pH 8, the silica tends to dissolve. Some higher purity columns can be used at higher pH, say up to pH 10, but will be expected to have shorter column lifetimes than at lower pH values. Special "hybrid" silica columns are designed for use at pH > 8. These columns actually use less-pure silica — a well-controlled addition of methyl or ethyl functional groups within the silica structure provides for a more inert particle. Once bonded with C18 or other stationary phases, hybrid particles can be used to pH 10 or sometimes higher with normal column lifetimes. Another option for high pH work is to use a column packing comprising polymeric particles instead of silica. These are very pH-stable, but often do not work quite as well from a chromatography standpoint as silica-based columns, so they are less popular.
Two red flags come to my attention with the stationary phase. First, is the silica one of the high purity or hybrid packings that can be used at pH > 8? Second, is the C18 bonded phase that was chosen sufficiently similar to the one used when the method was developed, so that the same separation can be obtained, especially in terms of the tailing factor?
Mobile phase: As mentioned previously, most C18 columns work best when the mobile phase is 2 < pH < 8. The specified pH was 9.6, which is well above the normal region. This will usually result in shorter column lifetimes, with shifting retention times and degraded peak shape over time. Another potential problem with high-pH work is that some of the silanol (-Si-OH) groups on the silica surface will ionize. These ionic sites add ion exchange to the dominant reversed-phase retention mechanism, often resulting in peak tailing. This means that peak tailing at high pH often is more common than at low pH.
To be effective, a buffer should be used within ±1 pH unit of its pKa The pKa of carbonate is 10.3, so buffer pH should not be an issue per se. However, ammonium carbonate is a volatile buffer. This makes it a common choice with mass spectrometry (MS) detection, or other evaporative detectors, because the mobile phase must be removed by evaporation. However, the volatility of carbonate buffer reduces its long-term stability, so many workers make fresh buffer every day to minimize buffer-related changes to the mobile phase. This might come as a surprise to users accustomed to phosphate buffers that are often used for 1–2 weeks without concern. Methanol is one of the two most common organic solvents used in reversed-phase LC today, so it is unlikely the source of the problem.
I am concerned about the age of the buffer and the effect of the high pH on column stability.
Column temperature: Those of you who read this column regularly know that column temperature is one of my "hot buttons". For isocratic separations, retention times can vary by 1–2% for each 1 °C change in temperature; the change is less dramatic for gradient separations, but is still important. In addition to retention shifts, selectivity often changes with temperature changes, especially if ionizable analytes are present. A change in temperature can change the mobile phase pH, the ionization of the analyte and the retention time; the combination of these can wreak havoc on reproducibility. Even if the laboratory temperature appears to be well-controlled, according to the thermostat, the microclimate containing the LC system can be much more variable, especially if a heating or air conditioning duct blows at the system. Operating the column at ambient temperature can generate some very surprising results when a method is transferred. Last year in January I visited a laboratory in China that was 10 °C and one in Tel Aviv in June that was 30 °C. Both used ambient column temperatures, but if our 2%/°C rule is applied, the retention times could vary by 20 °C × 2%/°C = 40%! I'm sure that the temperature of the laboratory in question does not change this much.
In spite of my concern about control of the column temperature, I don't think it is related to the current problem. The resolution is acceptable and peak tailing is unlikely to be affected strongly by normal changes in ambient temperature.
Sample preparation and injection: The sample preparation in the present method is very simple and there isn't much to go wrong. It is usually preferred to inject the sample with the mobile phase as a sample solvent, but with an injection volume of only 20 µL, this is unlikely to be a problem. So I wouldn't be concerned about this aspect of the method.
Sample retention: The retention time of the first peak is 2.5 min. We need to translate this into a k-value for a qualitative assessment. For this we need a value of t0 to use Equation 1; we should know t0 from the column test performed earlier. Or when 4.6 mm i.d. columns are used, we can estimate the column volume as another option. The volume (in milliliters) is approximately 0.01 L, where L is the column length (in millimetres). The 150 mm column's volume is ≈ 1.5 mL. This is converted to t0 by dividing by the flow-rate (1 mL/min) for a value of t0 ≈ 1.5 min. From Equation 1, k = (2.5 – 1.5)/1.5 = 0.67. For the second peak k = (3.7 – 1.5)/1.5 = 1.5. As a general guideline, I like to see k-values of at least 1 and preferably 2 or more for the first peak. Shorter retention times tend to cause the peak to be coeluted with interferences that come out at the solvent front and early peaks are much more susceptible to extracolumn effects and injection problems that can cause peak tailing. So it is possible that the short retention time might be contributing to the tailing problem. It would be easy to test this by reducing the organic content of the mobile phase by 5% from 57% methanol to 52%. This should increase retention, and if this were the problem source, would be an easy fix. Note that the short retention might not be due to a flaw in the method, but by using a different column than that used to develop the method. Different brands of C18 columns can cause retention times to differ by a factor of two or more under nominally identical conditions, although smaller retention changes are more common.
I don't like the small retention time of the first peak. This might or might not be the problem source, but would be easy to check by a simple mobile-phase adjustment.
Column lifetime: The user implied that the method was acceptable for the first ≈ 1000 injections before the tailing became too large to pass the system suitability test. Although column lifetimes of 2000 injections or more are common with simple drug product samples, such as this, 1000 injections is not unreasonable. If you consider the cost of a column, for example, $500, distributed over 1000 injections, it is only $0.50/injection for the column. In most laboratories, when all costs are considered, this is likely to be less than 1% of the cost of the analysis. At this point, the column has paid for itself and could be replaced without a significant impact on the cost of analysis. The concept of the column as a consumable item might seem strange at first, because of its high purchase price, but when compared to other consumable items in the chromatographic process, such as solid-phase extraction cartridges and even solvents, the column is not expensive on a per-sample basis.
I would like to see a longer column lifetime than was observed, but especially under the high-pH conditions, where column life is expected to be shorter, it might not be a major concern.
Now What?
We've made our list of potential problems in the system, now we need to figure out how to correct the problem. The potential problems cluster around the combination of the column and mobile phase. The high pH is expected to shorten the column life when compared to pH < 8; even the hybrid columns are expected to have shorter column life at pH > 8. As the column ages, the surface chemistry changes and peak shape changes often result, so increased tailing is not surprising. I would make sure that I was using the same column brand and model as was used in the original method, if this information is available. Otherwise, a high-purity silica or hybrid column would be the column of choice. Certainly the column temperature should be controlled, even though it is unlikely to be the problem in the present case.
Premature loss of buffer capacity due to the volatile buffer is easy to check by making a fresh batch of buffer — this is probably the first variable to check, because it is a simple test and if successful, could be incorporated in the routine use of the method. If a fresh buffer does not help and if a change in column does not correct the problem, or if the correct column is already in use, it might be worthwhile making a change in the mobile phase strength to see if larger retention times helps reduce the problem. Of course, this change might not be permissible for routine use of a validated method, but it could be tried on a one-time basis to see if it helped.
If this were my method, I would probably make a cursory evaluation of the variables, such as checking on the column specification and making up a new batch of buffer. If these did not solve the problem, I would live with the problem and replace the column more often. In the long run, it will be much less expensive to replace the column regularly than to make an involved investigation that might end in requiring revalidation of the method. The time and expense involved is probably not a good investment in most laboratory settings.
"LC Troubleshooting" editor John W. Dolan is vice president of LC Resources, Walnut Creek, California, USA; and a member of the Editorial Advisory Board of LCGC Europe. Direct correspondence about this column to "LC Troubleshooting", LCGC Europe, Park West, Sealand Road, Chester CH1 4RN, UK.
For an ongoing discussion of LC Troubleshooting with John Dolan and other chromatographers, visit the Chromatography Forum discussion group at www.chromforum.org

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












