Metabolome Studies of Herbal Medicine Using High Performance Liquid Chromatography Ion-Mobility Mass Spectrometry
In Chinese herbal medicine (CHM), traditionally, Hedyotis diffusa and Scutellaria barbata are cooked together and given to patients as tea. It is hypothesized that the interaction of metabolites from both herbs during cooking, improves the medical effect. This hypothesis was tested by preparing two tea variants which were then analyzed using high performance liquid chromatography (HPLC) coupled to ion-mobility mass spectrometry (IM-MS).
Africa Studio/stock.adobe.com

In Chinese herbal medicine (CHM), traditionally, Hedyotis diffusa and Scutellaria barbata are cooked together and given to patients as tea. It is hypothesized that the interaction of metabolites from both herbs during cooking, improves the medical effect. This hypothesis was tested by preparing two tea variants which were then analyzed using high performance liquid chromatography (HPLC) coupled to ion-mobility mass spectrometry (IM-MS).
According to the World Health Organization (WHO), 70–80% of the world’s population, or approximately five billion people living mainly in developing countries, are treated with herbal medicines as primary care (1). It is claimed that the combination of specific herbs results in mutual assistance, restraint, suppression, or antagonism leading to improved medical effects or a reduction of negative side effects (2). Possible modes of actions between the different herbs have been hypothesized (3). However, the impact of these interactions on the metabolite composition and on the medical effects of certain extracts remain unclear.
The annual herb Hedyotis diffusa (family Rubiaceae, also known as Oldenlandia diffusa) contains various important phytochemicals including iridoids, triterpenes, flavonoids, anthraquinones, steroles, cyclotides, coumarins, and alkaloids (4). The perennial herb Scutellaria barbata (family Lamiaceae chin.: “Ban-Zhi-Lian”) is one of the most important ingredients in Chinese herbal medicine (CHM). The dried aerial part of the plant is used for medical purposes. The compounds of Scutellaria barbata mainly comprise flavonoids, alkaloids, diterpenoids, and sugars. Important components known so far, as well as the current findings regarding their antitumour activity, are summarized and listed by Wang et al. (5). Both herbs are distributed in northeast Asia and commonly used in CHM for a variety of therapeutic activities including anti-cancer, anti-inflammation, and detoxification (6–10). In CHM, a mixture of both herbs is given to patients as a tea to treat different infections including hepatitis, sore throat, appendicitis, urethral infection, and certain cancer types (6–10).
The most commonly-used techniques for qualification and quantification of components in a complex sample are based on separation techniques such as gas chromatography (GC) or liquid chromatography (LC). However, using these methods alone can limit the separation of all components of a complex sample. To extend the separation power and provide more information on the separated compounds, multidimensional chromatographic techniques such as two-dimensional gas chromatography (GC×GC) or two-dimensional liquid chromatography (LC×LC) have been combined with different mass spectrometric (MS) techniques. A novel approach combines one-dimensional (1D-) or two-dimensional (2D-) high performance liquid chromatography (HPLC) with ion-mobility mass spectrometry (IM-MS). IM-MS allows the separation of ions according to their size-to-charge ratio as well as their mass-to-charge ratio (m/z). This enables the qualitative and quantitative detection of an increased number of components including isobaric species (11,12).
Experimental
In order to compare the metabolic compounds, two tea variants were prepared. For one tea sample, both herbs were cooked together, while for the other tea sample, the herbs were cooked separately and mixed together afterwards. Both tea samples were measured three times with a 6560 Ion Mobility QTOF Mass Spectrometer (Agilent Technologies) system coupled with a 1290 Infinity II UHPLC system (Agilent Technologies). The feature analysis was performed using IM-MS Browser B.07.01 software (Agilent Technologies). For detailed information, see Table 1.
Results
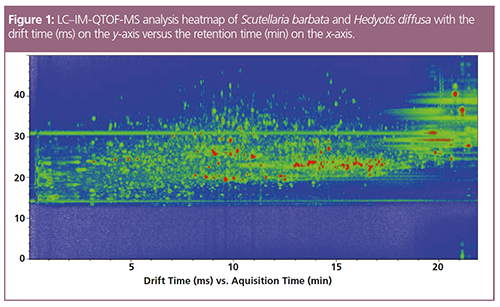
Figure 1 shows the heatmap of the HPLC-IM-quadrupole time-of-flight (QTOF)-MS analysis and demonstrates the separation power of HPLC coupled to IM-MS. Using the data obtained from the software for all six measurements (two tea sample variants with n = 3), a volcano plot was generated illustrating the feature volume differences between the two differently-prepared tea variants (Figure 2). This offers a quick overview on quantities of data.
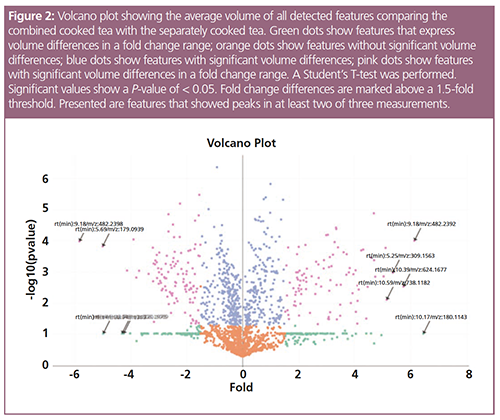
The location of each dot represents the relative volume of a detected feature. The volume describes the peak area sum of all ions created from one feature. The x-axis shows the abundance of each feature and the y-axis shows the statistical significance (P-value) of the abundance differences between separately cooked and combined cooked tea samples, respectively. Statistical significances were calculated using a Student’s t-test. The presented data demonstrate significant volume differences of around 200 features in a fold change range between both samples (only the determined features, which were found in at least two of the three different measurements, were used). These results strongly suggest differences in the “compound fingerprint” between the differently prepared teas and thus supports the hypothesis that the combined cooking of Hedyotis diffusa and Scutellaria barbata affects the compound composition and, in turn, the medical effects of the tea (2). Nevertheless, the correct detection of all peaks for every feature and every measurement has to be controlled manually in order to verify the results.
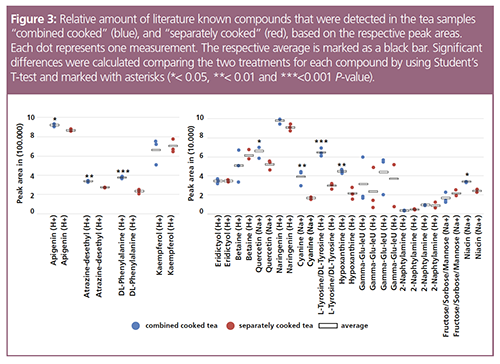
For further evaluation, the peak areas of several known metabolites were measured manually, compared, and statistically analyzed by performing a Student’s t-test. The results are presented in Figure 2. Some of the compounds detected in both samples show significant differences in the peak area (Figure 3). When both herbs were cooked together, the resulting tea contained a significantly larger amount of apigenin, atrazine-desethyl, DL-phenylalanine, quercetin, cyanine, DL-tyrosine, hypoxanthine, and niacin compared with the separately cooked tea. Flavones such as apigenin have long been described to have an anti-cancerogenic function. A lot of research has been performed in recent years on apenigin as a potential cancer prevention agent (13,14). For quercetin and kaempferol, mutualistic effects regarding their anti-cancerogenic activity have been described (15,16). Furthermore, niactin is reported to be important for genomic stability, with a great potential to reduce cancer risks (17). These results suggest that the combined cooking of Hedyotis diffusa and Scutellaria barbata is beneficial to the medical effects of the tea. Besides plant compounds, the synthetic compound cyanine and the herbicide atrazine-desethyl were detected and verified by collision cross section (CCS) values.
These results support our hypothesis that the combined cooking of Scutellaria barbata and Hedyotis diffusa causes changes in the metabolome composition of the herbal extract. The regulatory role of certain components on metabolome compositions and the underlying molecular mechanisms as well as potential changes in the medical effect remain to be elucidated in the future.
Conclusions
The results obtained by comparing the two samples clearly show significant differences regarding compounds and compound abundances. Scutellaria barbata and Hedyotis diffusa both contain large amounts of chemical compounds. Interaction of those compounds as well as the production of new compounds is therefore highly possible. The feature analysis revealed significant differences in the composition and abundance of features. The results obtained support our hypothesis, that the combined cooking of Scutellaria barbata and Hedyotis diffusa causes changes in the metabolome composition of the herbal extract. Non-target analysis has become an important tool in the field of complex samples, such as metabolomics, and food and beverage. For identification of compounds in such complex samples, often exact masses derived from high resolution mass spectrometric measurements coupled to liquid chromatography are used. The introduction of ion mobility spectrometry provides an additional separation dimension and allows the calculation of collision cross sections (CCS) of the analytes as a further physicochemical constant supporting the identification.
References
- World Health Organization Regional Office for the Western Pacific, Research Guidelines for evaluating the safety and efficacy of herbal medicines (WHO, Manila, 1993).
- K. Chan, Trends in Pharmacological Science16, 182–187 (1995).
- R. Yuan and Y. Lin, Pharmacology & Therapeutics86, 191–198 (2000).
- R. Chen, J. He, X. Tong, L. Tang, and M. Liu, Molecules21, 710 (2016).
- T.S. Wang, S.Q. Wang, and D.L. Xiao, J. Medicinal Plants Research6(26), 4259–4275 (2012).
- H.Z. Lee, D.T. Bau, C.L. Kuo, R.Y. Tsai, Y.C. Chen, and Y.H. Chang, Am. J. Chin. Med.39, 201–213 (2011).
- Q.X. Meng, R.H. Roubin, and J.R. Hanrahan, J. Ethnopharmacol.148, 229–238 (2013).
- Y. Niu and Q.X. Meng, J. Asian Nat. Prod. Res.15, 550–565 (2013).
- X. Kan, W. Zhang, R. You, Y. Niu, J. Guo, and J. Xue, BMC Complement Altern. Med.17(1), 41 (2017).
- Z.J. Dai, W.F. Lu, J. Gao, H.F. Kang, Y.G. Ma, S.Q. Zhang, Y. Diao, S. Lin, X.J. Wang, and W.Y. Wu, BMC Complement. Altern. Med.13, 150 (2013).
- C. Lipok, J. Hippler, and O.J. Schmitz, J. Chromatography A1536, 50–57 (2018).
- S. Stephan, C. Jakob, J. Hippler, and O.J. Schmitz, Analytical and Bioanalytical Chemistry408, 3751–3759 (2016).
- S. Shukla and S. Gupta, Pharm. Res. 27(6), 962–978 (2010).
- B. Sung, H.Y. Chung, and N.D. Kim, J. Cancer Prev.21(4), 216–226 (2016).
- G. An, J. Gallegos, and M.E. Morris, Drug Metab. Dispos.39(3), 426–32 (2011).
- M.L. Ackland, S. Van De Waarsenburg, and R. Jones, In Vivo19, 69–76 (2005).
- J.B. Kirkland, Mutat. Res.733, 14–20 (2012).
Oliver J. Schmitz is a full professor at the University of Duisburg-Essen in Germany and is the chair of the Applied Analytical Chemistry group. In 2009 he cofounded a company that develops new ion sources and units to couple separation techniques with mass spectrometers. He is interested in the development of ion sources, the use and optimization of comprehensive LC, and coupling analytical techniques with ion mobility mass spectrometers. He was awarded the Gerhard-Hesse Prize for chromatography in 2013 and the Andrzej Waksmundzki Medal Award for Analytical Chemistry at the Polish Academy of Sciences and the Polish Chemical Society in 2018.
Lisa Mahdi obtained her Master’s degree in biology at the University of Cologne and the Max Planck Institute for Plant Breeding Research Cologne (both in Germany) in 2018. Her interests include molecular plant-microbe interactions and cell death. The main objective of her Ph.D. is to characterize molecular mechanisms underlying root-microbe multispecies interactions and cell death regulation during the accommodation of beneficial and pathogenic microbes in the root.
E-mail: oliver.schmitz@uni-due.de
Website:www.uni-due.de/aac and www.oliver-schmitz.net
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

