Meeting the Updated Requirements for the Determination of Residual Solvents in Pharmaceutical Materials
The Application Notebook
The United States Pharmacopeia (USP) has implemented a revised method for the determination of residual solvents, chapter 467; this revision has brought the methodology of USP 467 into close alignment with European Pharmacopeia (EP) method 2.4.24. The USP and EP determination of class 1 and class 2 residual solvents is performed with static headspace (HS) sample introduction and gas chromatography (GC) with flame ionization detection (FID); class 3 has flexibility in the technique, however, it is often included in the HS analysis.
The United States Pharmacopeia (USP) has implemented a revised method for the determination of residual solvents, chapter 467; this revision has brought the methodology of USP 467 into close alignment with European Pharmacopeia (EP) method 2.4.24. The USP and EP determination of class 1 and class 2 residual solvents is performed with static headspace (HS) sample introduction and gas chromatography (GC) with flame ionization detection (FID); class 3 has flexibility in the technique, however, it is often included in the HS analysis.
The purpose of this paper is to work under the guidance of the USP and EP to achieve the best method possible for the analysis of residual solvents, while adhering to USP 467 and EP 2.4.24 criteria. Discussed will be the analysis of class 1 residual solvents.
Experimental
The instrumental platform used in these analyses, was the PerkinElmer® TurboMatrix™ HS-40 headspace system and the PerkinElmer Clarus® 600 Gas Chromatograph with FID. The following instrument parameters used in this study achieved results which exceeded the USP detection-limits method criteria for the analysis of class 1 and class 2 solvents.
The sample vial was equilibrated at 80°C for 20 minutes; the needle temperature and transfer line temperature were maintained at 110°C and 140°C, respectively. The injection system utilized was the pressure-balanced sampling technique. The system first pressurizes the headspace vial for one min at 18 psi. Following pressurization, the vial pressure is allowed to decay onto the column performing a timed sample injection. The time used was 0.1 min which allowed for a 1-mL volume of headspace to be injected.
The pressure-balanced sampling technique allows for optimum analytical results, including enhanced precision, recoveries, detection limits, and inertness.
Within USP method 467, there are two sets of column/oven conditions, A and B. The data presented here were acquired utilizing the GC conditions described in procedure A.
Results
The measure of system suitability for class 1 residual solvents is determined by the signal-to-noise value for each component. When analyzing class 1 solvents by procedure A, the criteria of signal to noise for benzene must be greater than 5:1 and the signal to noise of the other components must be greater than 3:1.
The experimentally determined signal-to-noise values are presented in figure 1; the signal to noise achieved ranged from 11:1 for carbon tetrachloride, a poorly-responding component when FID detection is used, to 143:1 for 1,1-Dichloroethene, with benzene responding at 89:1.
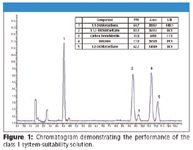
Figure 1
Conclusion
Demonstrated here are method and data which operate under the guidance of and fulfill the performance criteria of both USP 467 and EP 2.4.24 for the determination of residual solvents in pharmaceutical materials. Recent revisions of these methods have aligned the analytical techniques to allow for a single method to meet the criteria of both organizations. Within the scope of USP chapter 621 and EP chapter 2.2.28, the injection volume was reduced to improve peak shape and method performance; necessary sensitivity was maintained with the modified method.
PerkinElmer, Inc.
940 Winter Street, Waltham, MA 02451 USA
Tel.: 800-762-4000 or (+1) 203-925-4602
Fax: 203-944-4904


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













