Meeting Regulatory Needs in the Characterization of Lipid Nanoparticles for RNA Delivery via FFF-MALS
A validated FFF-MALS method aligned with technical specification ISO/TS 21362 for the analysis of lipid-based nanoparticles (LNPs) encapsulating siRNA and mRNA is presented.
Field-flow fractionation coupled to multi-angle light scattering (FFF-MALS) is a powerful analytical approach for the advanced characterization of nanomaterials (sizes < 1 µm). Contrary to size-exclusion chromatography (SEC), no stationary phase is required during the fractionation process, and the optimal separation range can be fine-tuned by varying flow parameters. This flexibility enables reliable measurement of complex pharmaceutical products, such as nanomedicines, monoclonal antibodies (mAbs), and vaccines. For quality control purposes, the FFF-MALS protocol must satisfy regulatory needs and accepted standards. This article describes a validated FFF-MALS method aligned with technical specification ISO/TS 21362 for the analysis of lipid‑based nanoparticles (LNPs) encapsulating siRNA and mRNA.
Lipid-based nanoparticles (LNPs) for nucleic acid delivery, especially for short interfering RNA (siRNA) and messenger RNA (mRNA), have recently attracted extraordinary attention, and are expected to revolutionize the medical field. At the end of 2020, a milestone was reached with two vaccines against the ongoing COVID-19 pandemic, based on mRNA strands encapsulated in LNPs, approved by regulatory authorities in the USA and Europe: the BioNTech/Pfizer’s tozinameran and Moderna’s mRNA‑1273. Apart from COVID-19 vaccines, further nucleic acid‑based therapies are in development for a broad range of applications spanning immune‑modulating agents, protein replacement therapies, regenerative medicine, and gene‑editing complexes, amongst others. Nanocarriers such as LNPs can protect active pharmaceutical ingredients (APIs), enhance bioavailability, and thus improve safety and efficacy of novel therapies (1).
The use of lipid nanocarriers to deliver nucleic acid increases the complexity of the formulation and consequentially introduces the need for sophisticated analytics to ascertain a stable and safe drug product. Based on guidelines for drug products containing nanomaterials, including liposome characterization, the following parameters can be regarded as plausible critical quality attributes (CQAs): particle concentration, particle average size and polydispersity, nucleic acid loading levels, and chemical stability and physical stability (aggregation propensity). Clearly, the advancement of robust analytical methods for CQA determination that are compliant with regulatory requirements is essential to streamline development and quality control.
Field-flow fractionation (FFF) physically separates species of different sizes or,
more precisely, hydrodynamic volumes (Figure 1). First, the unseparated sample
is injected into the channel. In conventional channels, the sample attains equilibrium height above the membrane in a focusing step; this action tends to concentrate the sample in close proximity to the membrane. The dispersion inlet channel (also known as frit inlet channel) uses the “hydrodynamic relaxation” principle for which no focusing step is required, diminishing unwanted effects of high local concentrations. A parabolic channel flow then transports the sample towards the channel outlet. Since small particles have a higher diffusion coefficient, they will on average be higher above the bottom membrane and hence exposed to a higher flow rate. Thus, small particles elute first, followed by larger species, inversely to the order in size‑exclusion chromatography (SEC).
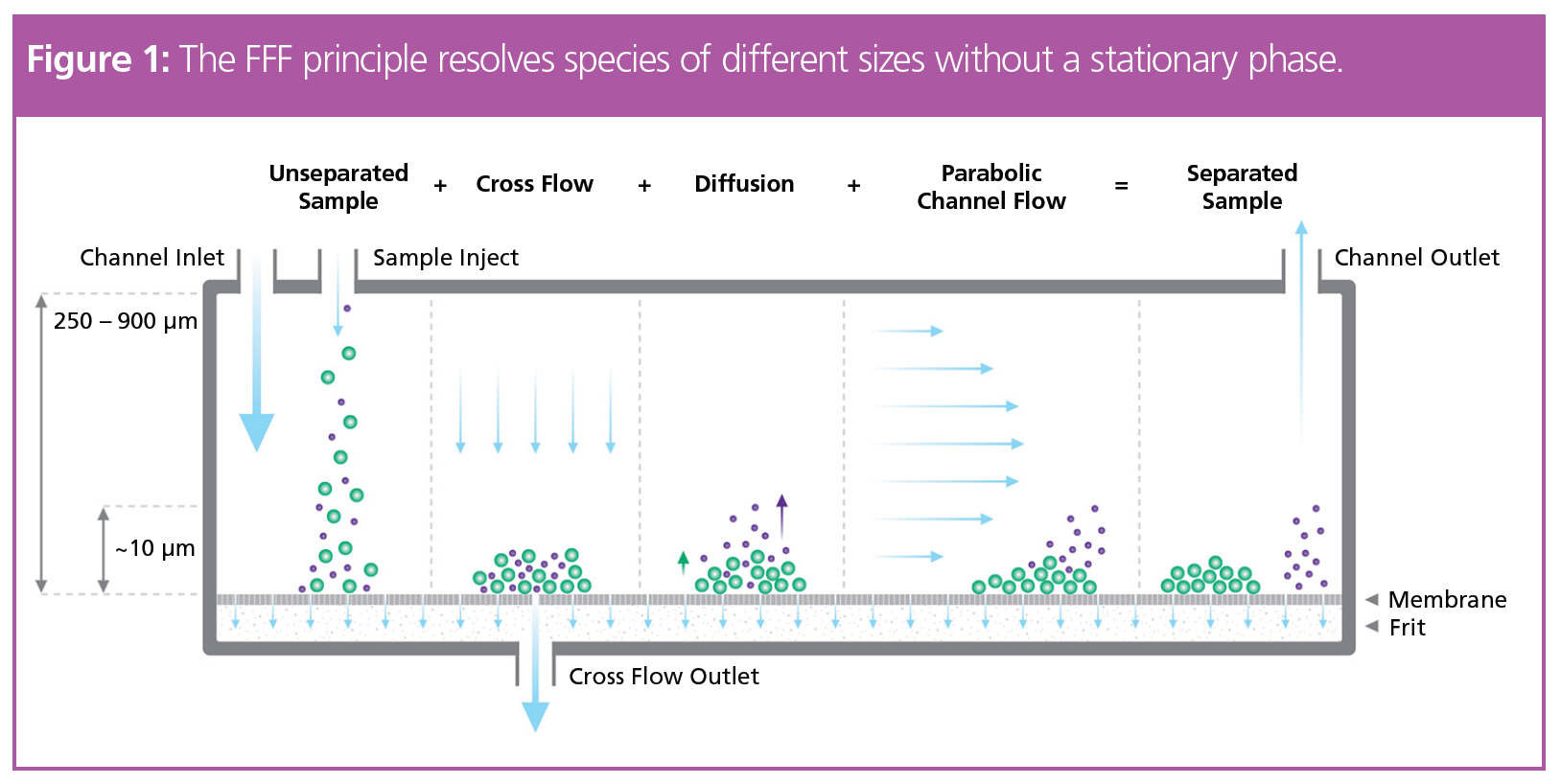
The particle retention time, tr, is directly proportional to the hydrodynamic radius, Rh, and the tunable cross flow/channel flow ratio. Subsequent multidetector analysis including UV, multi-angle light scattering (MALS), online dynamic light scattering (DLS), and differential refractive index (dRI) detection elucidates several key sample parameters in the same measurement. This was recently demonstrated for pharmaceutical products including liposomal drug formulations (2,3). Based on the 2021 publication by Mildner et al. (4), we outline here the establishment of a robust FFF‑MALS method for the characterization of lipid nanoparticles encapsulating RNA (RNA‑LNP). Key parameters that can be measured with FFF-MALS are average particle size, polydispersity, morphology, physical stability, and particle concentration.
To fulfill regulatory requirements, the repeatability, reproducibility, and robustness of the analytical methods used to characterize a drug product need to be addressed according to the harmonized guideline ICHQ2R1 (5). Even if ICHQ2R1 addresses mostly classical chromatographic methods and does not specify the requirements for sizing measurements such as FFF-MALS, ISO/TS 21362 (6) could be used as a suitable reference for the validation of FFF‑MALS methods. The following criteria need to be met:
- Recovery of the analyte ≥ 70% (performance separations, such as particle concentration, would require recovery ≥ 90%);
- Relative standard uncertainty ≤ 5% for retention time, recovery, and particle size values.
This article describes FFF method development, including channel-type selection, and demonstrates the powerand versatility of FFF-MALS for the characterization of siRNA-LNP and mRNA‑LNP according to regulatory needs.
Experimental
The experimental procedure is described in depth in reference 3. In brief, the samples were analyzed using an Eclipse FFF instrument (Wyatt Technology) operated with an isocratic pump, degasser, and autosampler from the 1260 Infinity II series (Agilent Technologies). Fractionation was performed in the Eclipse conventional long channel and dispersion-inlet channel. Sample recovery was calculated by integrating the main UV peak area at 260 nm for each sample, with and without the applied cross flow and focusing step.
Detection was accomplished by a DAWN MALS instrument with embedded DLS module (Wyatt Technology). Data were collected and analyzed in Astra software (Wyatt Technology) to determine particle size and concentration. Further data processing calculated the particle size distribution in particles/mL/nm for 2 nm bin sizes (4).
High Recovery as Key Measurement Quality Indicator
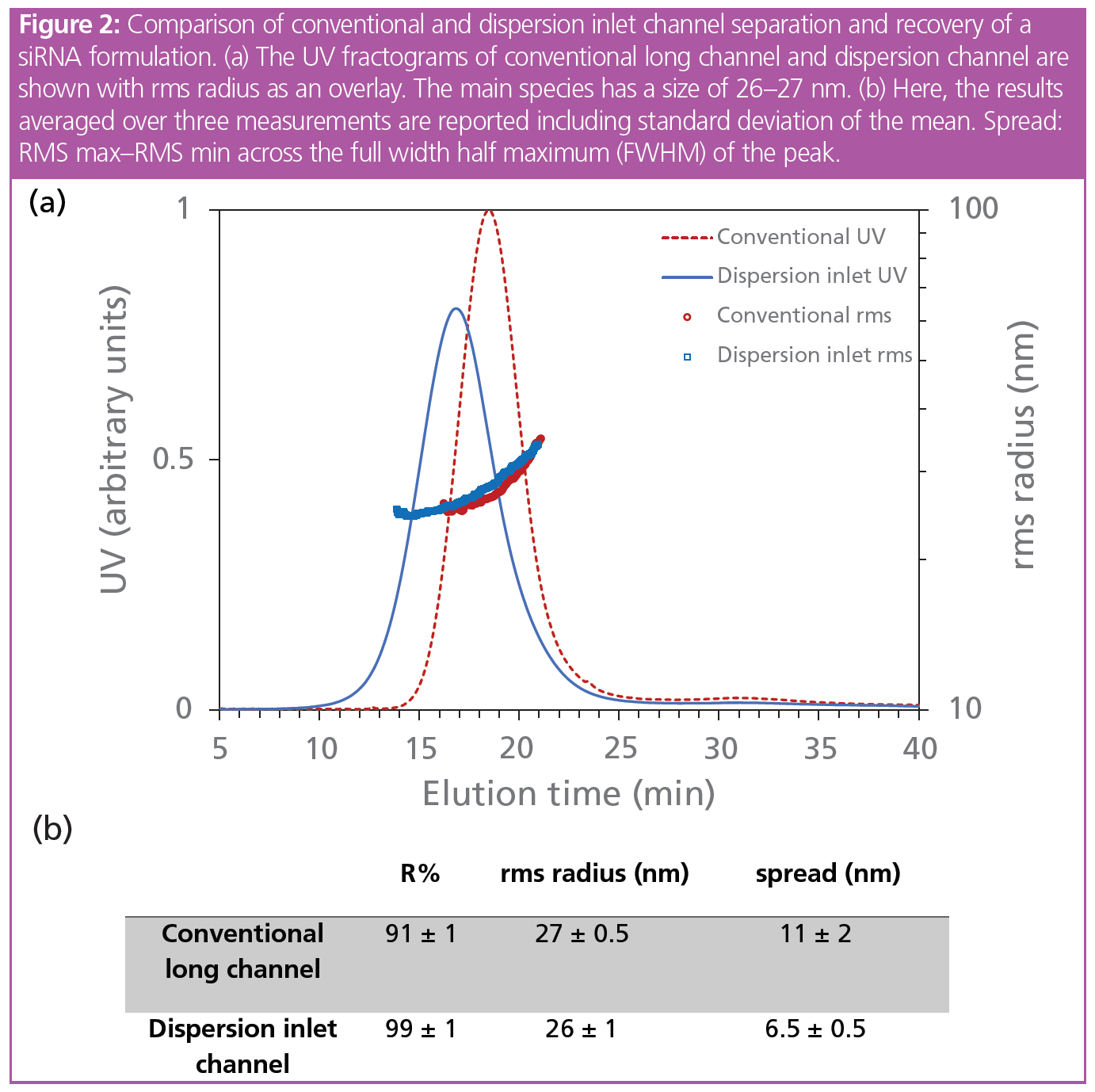
Sample recovery (R%) is the key parameter to consider for initial method optimization. It is determined by main UV peak integration with and without cross flow and focusing step. During method development, the conventional setup with a standard FFF long channel was compared to a method using the dispersion inlet channel, where the sample is injected directly into the carrier stream. Here, no focusing is required, reducing the risk of sample destabilization and increasing recovery for delicate samples such as LNPs. Figure 2 shows the results obtained by measuring the same siRNA‑LNP formulation by using the two channels. In both channels the sample is eluted and fractionated according to the particle size. Although in the tested configuration both channels fulfill the quality criteria in terms of R%, the dispersion inlet method was used for advanced characterization of LNPs due to the higher recovery and its benefits for sensitive samples.
Determination of Size, Morphology, and Particle Concentration by Using an Optimized Dispersion Inlet Method
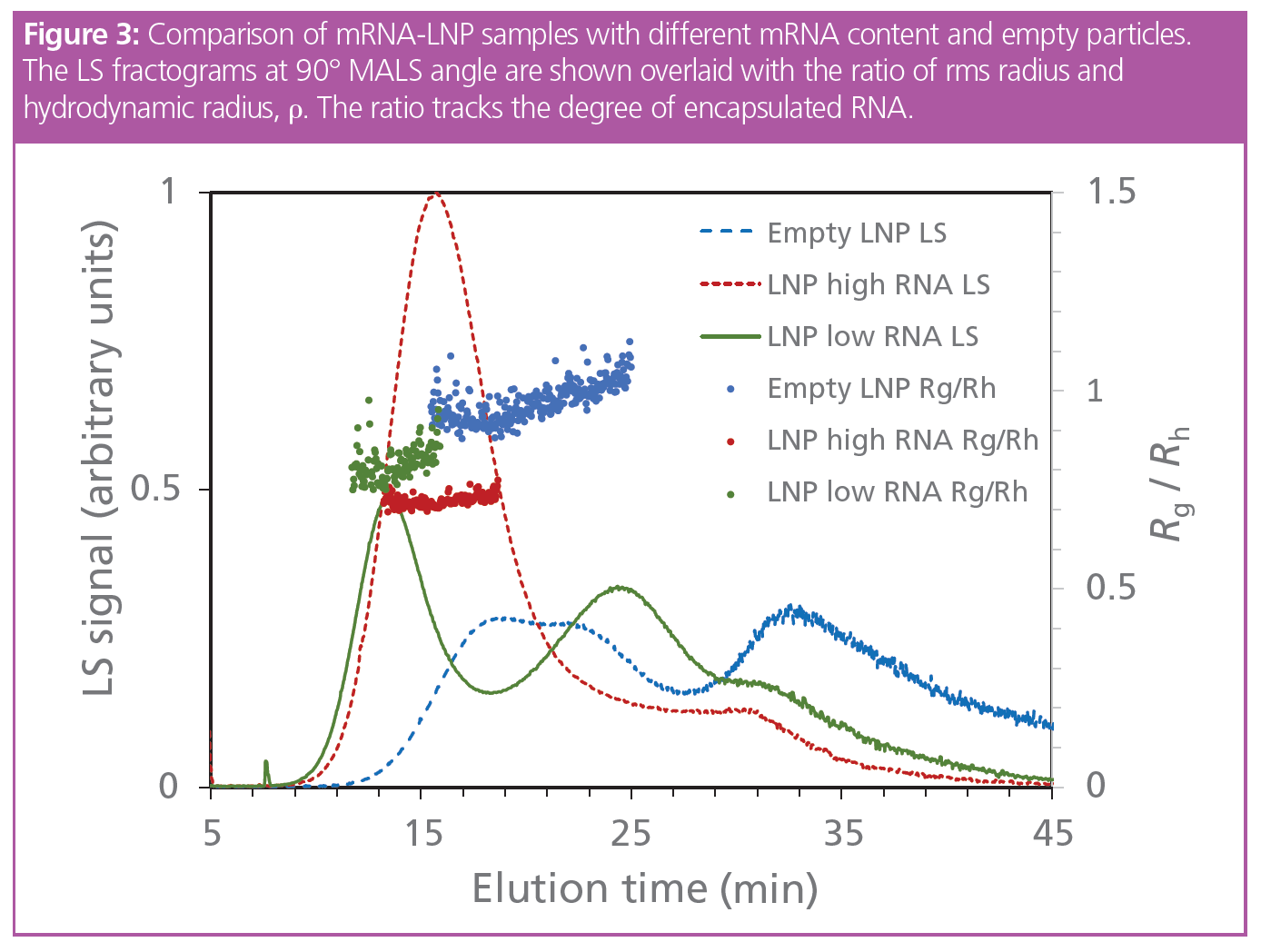
Using the optimized fractionation method, with MALS and DLS detectors online, several LNP key attributes can be determined, including: (i) average size and polydispersity, (ii) particle morphology, and (iii) particle concentration. As proof of concept, three formulations with variable mRNA payload were measured to determine both the sample size, polydispersity, and particle morphology: empty particles, particles with low (N/P = 8) RNA content, and particles with high (N/P = 3) RNA content. The particle size and polydispersity were higher for lower mRNA content, and even more pronounced for empty particles, indicating that a minimum amount of mRNA is needed to obtain a monodisperse formulation. In fact, it is known that in the absence of a sufficient amount of RNA, the LNP nanostructure where the mRNA is complexed by the ionizable lipids and the other particle components cannot be formed.
Differences in particle size and polydispersity are therefore associated with differences in particle morphology, another important parameter that can be determined by FFF-MALS. MALS with simultaneous online DLS to determine hydrodynamic radius (Rh) elucidates particle morphology (shape, core density) associated with the RNA loading. For a hollow sphere, ρ (the ratio of rms radius [determined by MALS] to Rh) is unity. For a sphere with a dense core, however, the scattering centres are distributed closer to the centre of mass and ρ ≤ 0.77 is expected. Figure 3 shows ρ vs. retention time of mRNA-LNP samples with different RNA content, and of the empty LNP control sample. Interestingly, the empty particles possess ρ ≈ 1, typical of a hollow or irregular sphere, while increasing mRNA contents reduces the ratio. The value of the monodisperse mRNA particles match the ratio associated with a dense spherical particle. Note, the empty LNP and the mRNA particles loaded with a lower payload show a larger proportion of aggregates, and are less stable, showing the correlation between particle morphology, monodisperse size distribution, and the physical stability of the LNP formulations.
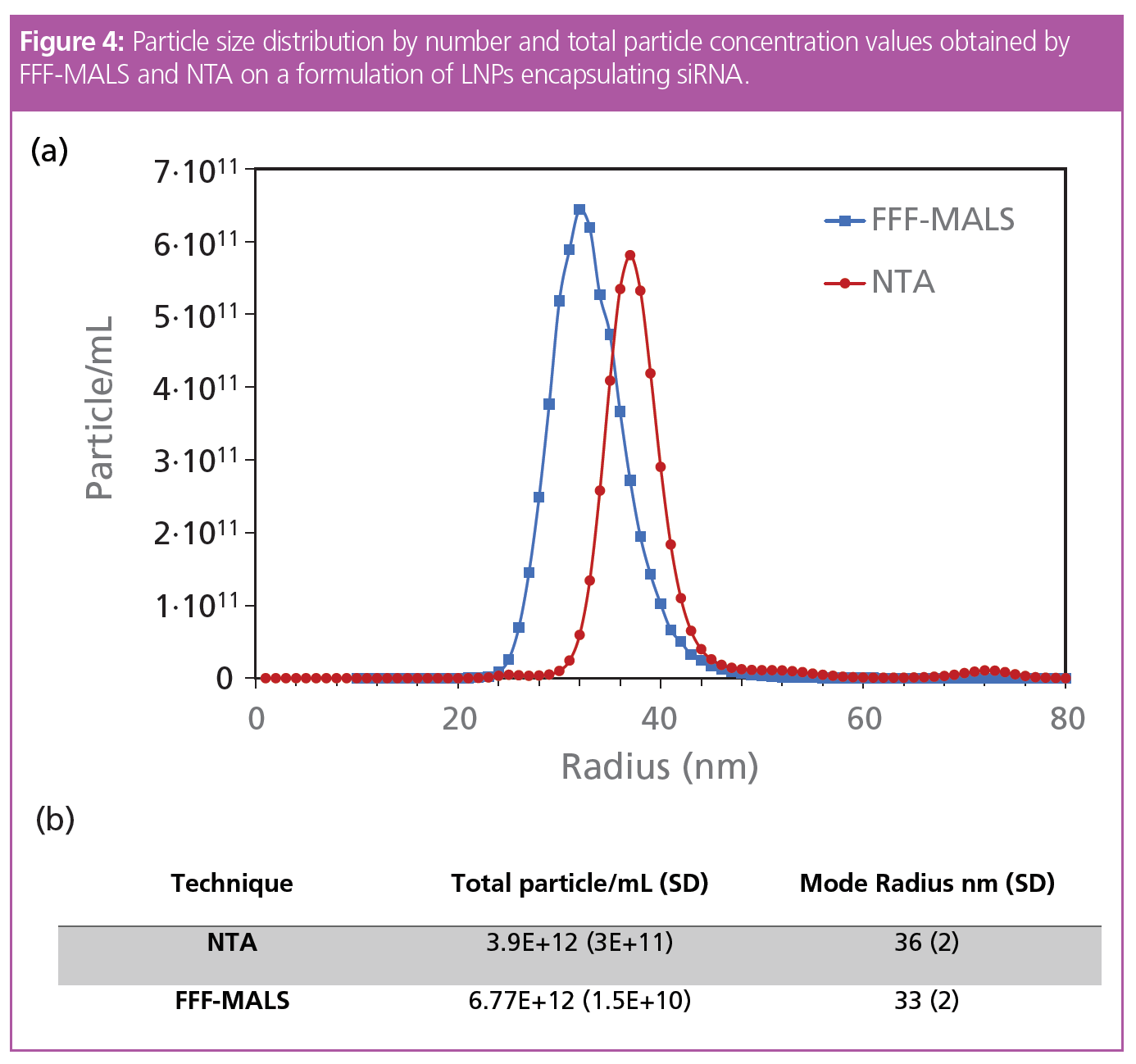
As demonstrated by Mildner et al. (4), the data obtained by MALS, in combination with knowledge of particle shape, structure, and refractive indices of the particles and solvent, determine particle concentration and thus derive the number-based particle size distribution. As a proof of concept, the results obtained by FFF-MALS were compared with particle concentration measured by nanoparticle tracking analysis (NTA) and showed remarkably comparable results (Figure 4). Notably, FFF-MALS detects and quantifies LNP particles below 30 nm in radius that are too small for NTA.
LNP Stability Assessment
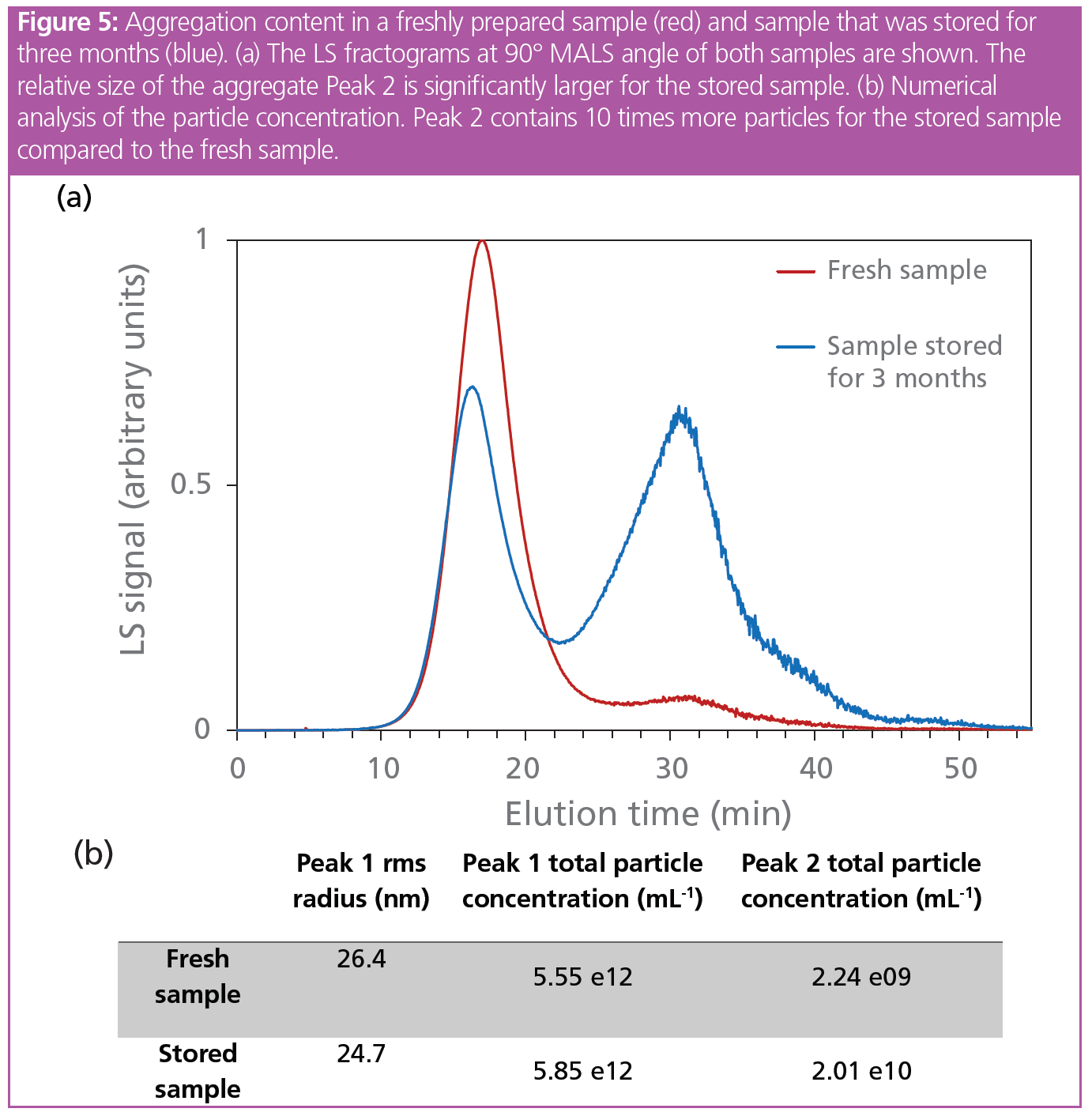
A critical parameter for RNA-LNPs is their stability. As FFF-MALS can determine size, morphology, and concentration with high resolution, it is one of the methods of choice for evaluating the physical stability of the particles, and to determine their tendency to aggregate. Here, a freshly prepared LNP sample loaded with siRNA was compared to a sample prepared three months earlier and stored at 4 °C. The freshly prepared sample showed one main particle population (Peak 1: Rt= 17 min) and a small number of larger particles eluted as a second peak (Peak 2: Rt = 31 min). After 3 months of storage, the size associated with the main population was slightly decreased, while the intensity of the peak associated with larger particles and/or aggregated was significantly higher (Figure 5). The change in the particle size distribution and substantial increase in sample polydispersity is called Ostwald ripening and is induced by the migration of lipids and particle components from smaller particles to larger ones. Higher sample polydispersity immediately following synthesis corresponds to faster Ostwald ripening and thus larger particle instability. This highlights the power of FFF-MALS to study changes in size, polydispersity, and concentration of LNP formulations over time.
Conclusion
Thorough method development and validation are crucial to move FFF-MALS into standard QC procedures in the nanomedicine field. Here, we demonstrated method development following the ISO/TS 21362 technical specification and showed results for RNA-LNP. We presented a robust FFF-MALS approach that can be used by the pharmaceutical industry to characterize nucleic acid-based nanotherapeutics during drug development and for quality control purposes. Importantly, FFF-MALS measures multiple CQAs of LNP nanoparticles encapsulating mRNA or siRNA, such as particle size distribution, particle morphology, and particle concentration, as well as physical stability and aggregation propensity.
References
- I. Gómez-Aguado et al., J. Nanomat.10, 364 (2020).
- J. Parot et al.,J. Control Release 320, 495–510 (2020).
- F. Caputo et al., J. Chromatogr. A1635, 461767 (2021).
- R. Mildner et al., Eur. J. Pharm. Biopharm. (in press) (2021).
- International Council for Harmonisation, ICHQ2R1, Validation of Analytical Procedures: Text and Methodology (ICH, Geneva, Switzerland, 2005) https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
- https://www.iso.org/obp/ui/#iso:std:iso:ts:21362:ed-1:v1:en
Fanny Caputo is a research scientist at SINTEF (Norway). Her main interests lie in the physical-chemical assessment of nanomaterials, nanoplastic, medical devices, and pharmaceutical products containing nanomaterials, including lipid-based nanoparticles for nucleic acid delivery.
Christian Sieg works as an application scientist for Wyatt Technology and is responsible for instructing customers to utilize Wyatt’s instrumentation. His work includes visiting customers onsite, establishing applications, and training.
Website: www.wyatt.com/LNP

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













