Mastering Pressure Management in GPC/SEC Systems
This installment of Tips & Tricks deals with pressure monitoring for GPC/SEC systems and gives hints for efficient troubleshooting to solve issues that are identified in the laboratory.
Excessive pressure or unexpected pressure fluctuations are common issues in liquid chromatography (LC). Gel permeation chromatography, also known as size-exclusion chromatography (GPC/SEC) is no exception to that rule. GPC/SEC often involves the use of polymeric-based columns, which are significantly less pressure-stable than traditional silica-based HPLC columns. Therefore, being aware of pressure changes in GPC/SEC systems is crucial, especially considering the risk of irreversibly damaging the GPC/SEC columns if the pressure is too high.
Although technical solutions exist, such as setting an upper pressure limit for the pump to protect the columns, or using an internal flow marker (1), it is nevertheless advisable to actively monitor the pressure when running samples. Modern instruments often provide the pressure as an independent trace, which can be recorded. When encountering strange chromatograms or shifts in elution volumes, reviewing this data set can help to identify the problems and even provide hints for their potential solutions.
Before Getting Started With GPC/SEC
To ensure smooth operation of the GPC/SEC, it is essential to be familiar with at least two different pressure values and their normal operating conditions, considering setup, flow rate, and temperature:
- Equipment Pressure (no columns): Measure the pressure of the equipment when the injector outlet capillary and the detector inlet capillary are connected using a low-dead-volume union, without any columns installed.
- GPC/SEC System Pressure (all columns installed): When combining multiple columns in a column bank, check the pressure after each single column (including the precolumn) is installed.
Knowing these reference values simplifies diagnosis when a pressure change occurs to troubleshoot pressure variations, disconnect the columns, reinstall the low-dead-volume union, and operate the system. If the pressure with the union installed remains the same, the columns are likely causing the pressure change.
Expected Behavior of the Pressure Trace
The absolute value of the pressure itself depends on many factors such as column characteristics, system, and method parameters.
The contribution of the columns to the overall pressure depends on several factors: the number of columns installed (the total length of the column bank), the columns’ inner diameter, and the stationary phase particle size.
Examples of system parameters contributing to the pressure value include the length and inner diameter of the capillaries, the number of detectors attached, their cell designs, and the use of inline filters—for example, post-column filters or frits.
The flow rate is the method parameter contributing most to the absolute pressure value. However, the viscosity of the mobile phase, as well as the temperature in the column compartment and the laboratory, also influence the absolute pressure value.
Figure 1 shows the pressure trace of a well-maintained GPC/SEC system comprising an autosampler with flow-through injection needle and injection through the needle seat. The recorded pressure trace allows identification of the single steps during injection (identified by the black straight line and the blue triangle on the x-axis) and provides valuable insights.
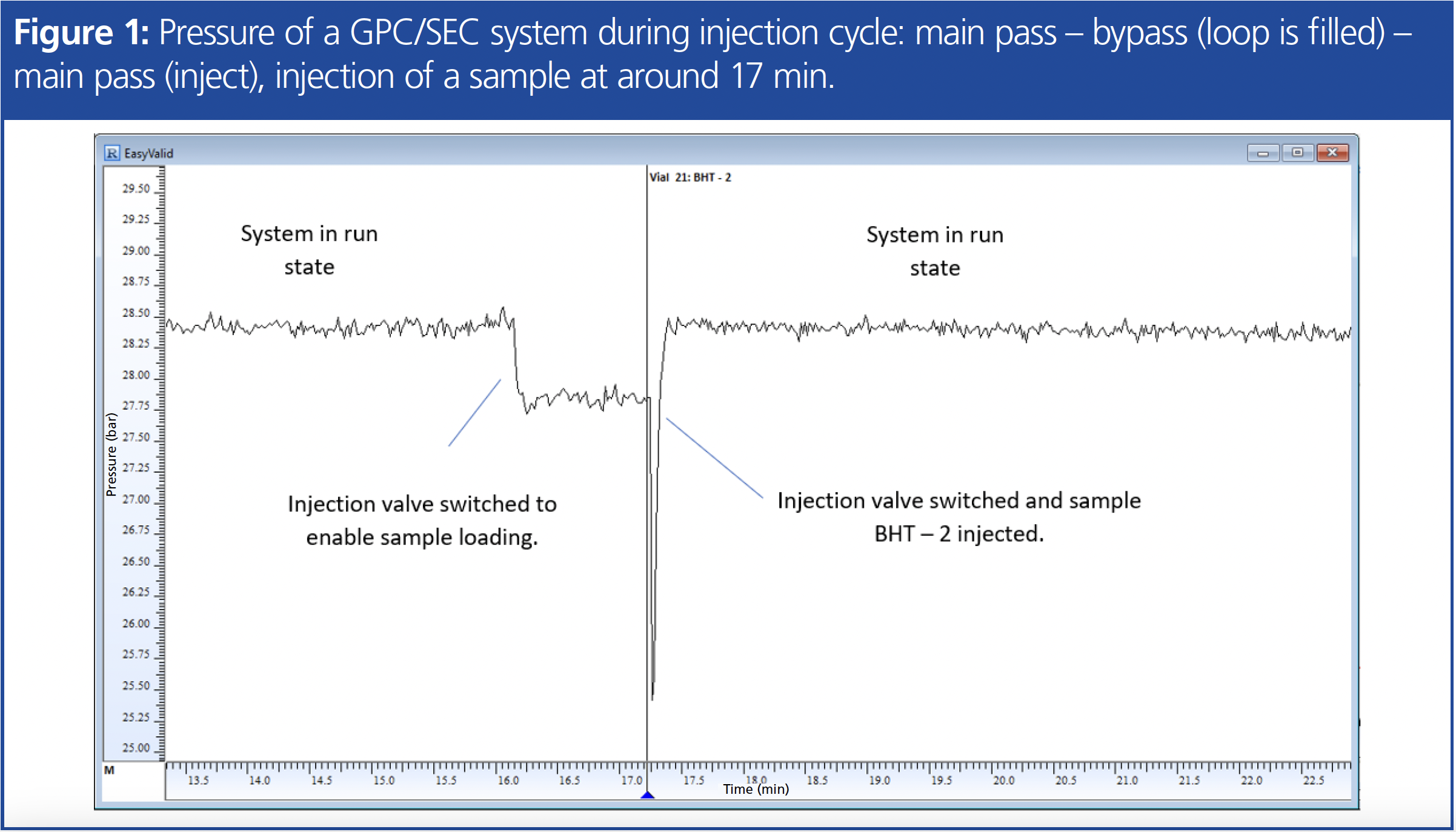
The pressure is seen to be stable and without large fluctuations. The subsequently observed pressure drop corresponds to switching the autosampler injection valve from injection mode (main pass) to sample loading mode (bypass). The pressure remains stable until the sample is loaded and the injection valve returns to its initial position. As the sample is injected onto the column, the pressure quickly returns to its previous level, as expected.
It’s important to note that for autosamplers without inline injection systems, the trace would appear differently. However, in all cases, a pressure change followed by a rapid return to the original level after sample injection should be observed.
Typical Observations and Their Potential Root Causes
When monitoring the pressure during analysis, it is quite easy to distinguish between different incidents. The pressure trace will show what has happened and when it happened.
The following observations are typical scenarios:
a) Abrupt and Immediate Pressure Decrease: In the case of an abrupt and immediate decrease in pressure, the root cause can be either air in the system, a broken detector cell, a significant leak, or a faulty or unreliable inlet or outlet valve on the pump.
Temperature changes in the laboratory or in the column oven can also potentially decrease the system pressure but this will generally be a more subtle, less abrupt change. For more viscous mobile phases such as dimethylformamide (DMF), dimethylacetamide (DMAc) or dimethyl sulfoxide (DMSO), this change will be more pronounced.
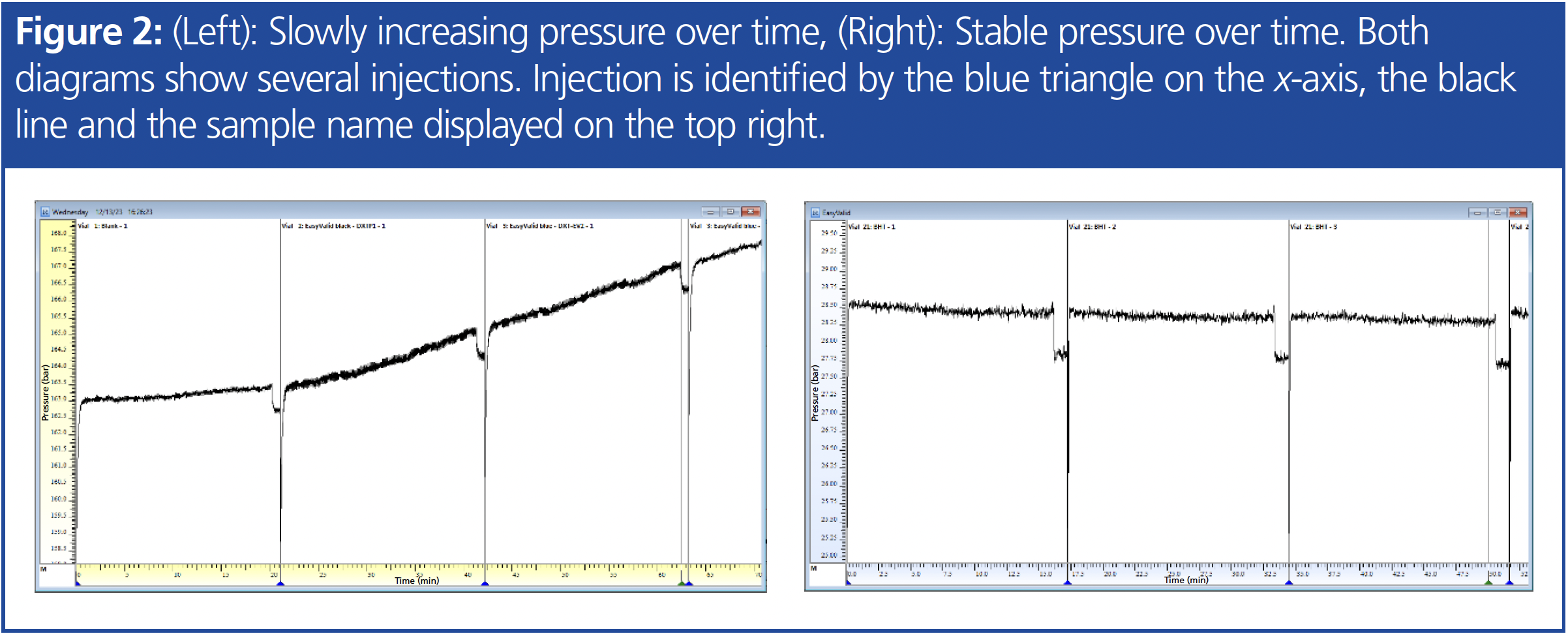
b) Constantly Increasing Pressure Over Time: A consistently increasing pressure is usually attributed to a capillary or frit that gradually becomes clogged. Additionally, issues with the column or the column bank may contribute to this phenomenon. In Figure 2, the left side illustrates a trace of pressure gradually rising over time, while the right side shows a stable pressure.
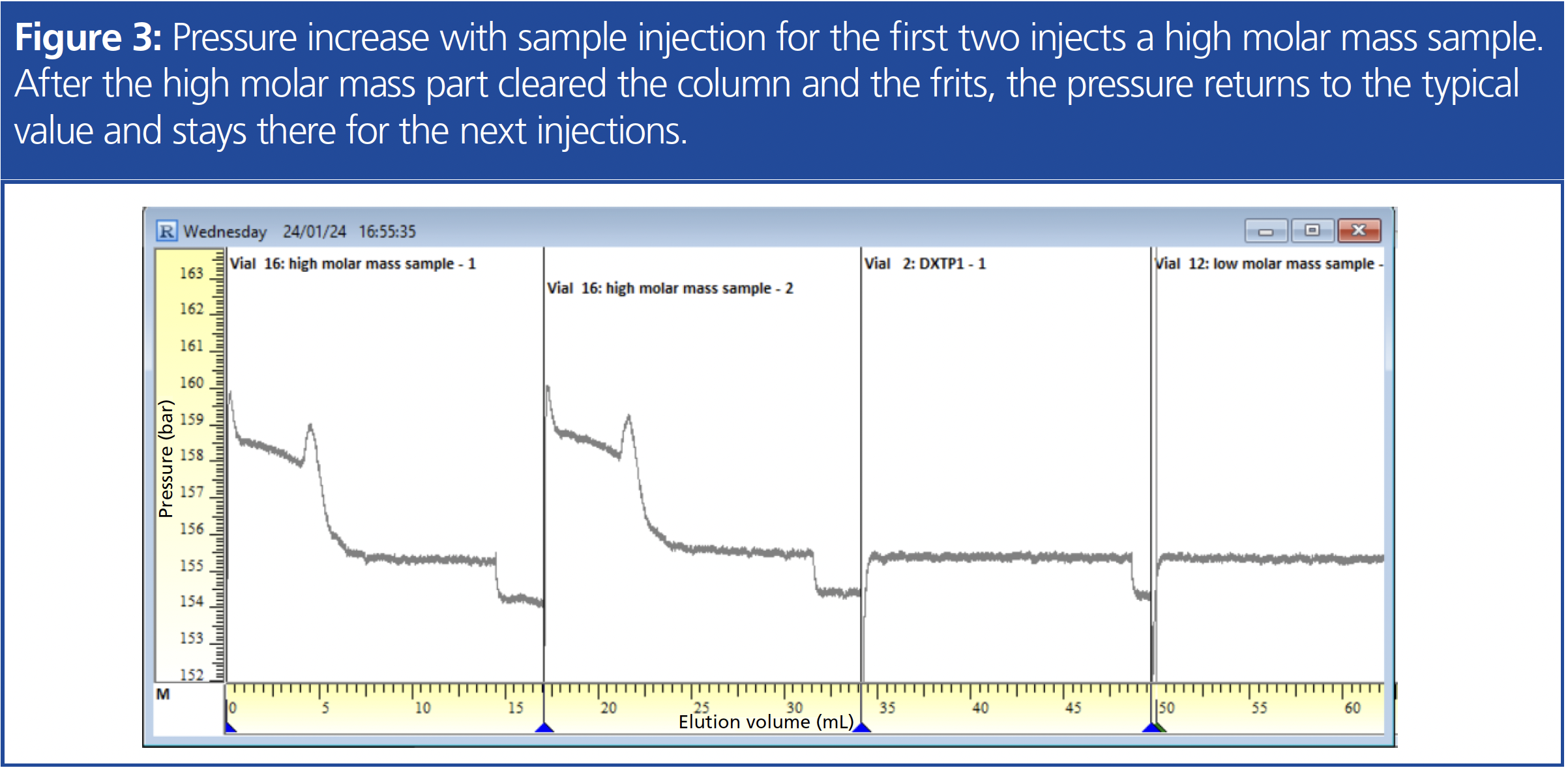
c) Abrupt Increase in Pressure: If an abrupt increase in pressure is observed, this is often related to problems with sample injection. Potential sources can be blockages in the injection needle, the injection valve, or (if present) the needle seat. For example, it could be that fragments of an unsuitable vial cap septum got stuck in the injection system. Additionally, it could be caused by insoluble parts of the sample getting stuck in the system or on the column frits, or, in the worst case, it could be caused by a sample interacting with the column stationary phase. In all these cases, the pressure increase will be permanent, and the pressure will not return to its original value.
Figure 3 shows a very interesting case where the pressure increases with the injection of a specific sample but returns to normal once the highest molar mass part of the sample has cleared the column. This is observed for both injections of this sample (each indicated by the blue triangle at the bottom and the dark straight line with the sample name at the top right). For the following two injections, both lower molar mass samples, no pressure increase is detected. If a temporary pressure increase, such as this example, is observed with a sample, a review of the sample preparation is recommended. The concentration might be too high for the molar mass range, leading also to a high viscosity. Special precautions for high molar mass samples can help to minimize this effect (2).
Overcoming Observed Pressure Problems
When encountering issues with ambient temperature GPC/SEC systems, it is advisable to first deinstall the GPC/SEC columns, securely plug them, and store them in a safe place until the problem is resolved or until further column inspection is necessary.
a) Addressing Abrupt Pressure Decrease:
- If you encounter an abrupt and immediate pressure decrease, start by examining the mobile phase reservoir. Look for air trapped in tubing. It can be removed by opening the pump purge valve and operating the pump at high flow rates. Consider using a syringe to thoroughly flush the pump head with solvent if necessary.
- If the pump is filled with mobile phase, the waste tubing of the purge valve should be monitored carefully. Is solvent dripping from it at a constant rate? If not, there might be a problem with the inlet valve of the pump.
- Close the purge valve and monitor the pressure of the system. It might be necessary to install a restriction capillary to achieve a higher backpressure so that the pump is in its stable operation mode. Frequent pressure drops could indicate a problem with the outlet valve.
- Finally, check for leaks. Inspect all connections, including the injection valve and the detector cells, and pay special attention to any potential leaks.
b) Addressing Pressure Increase:
- If you encounter a sudden pressure increase, start by reinstalling the low-dead-volume union, especially if you know the original setup value. Compare the old and new pressure values to determine whether the columns or GPC/SEC equipment are responsible for the incident. If the pressure is too high, further inspection will be necessary.
Case 1: Equipment Checks
Equipment parts that can be easily checked are any filtering units and frits, for example, inside the purge valve of the pump, or perhaps installed separately as postcolumn filters.
Another quick test is to switch the injector between injection and loading positions when the pump is running. This helps to identify blockages in the injection valve and related parts. If the pressure remains too high when the valve is in the injection position, check and replace the capillaries and single components, such as the needle or the injection valve rotor seal.
If these steps do not identify the source of the problem, other capillary connections can be opened one after the other, preferably while the pump is set to a lower flow rate. However, the pressure must still be high enough so that pressure drops can be easily monitored if a connection is opened. The connection does not have to be opened completely; just loosening the connection by a couple of full turns can already be successful. As well as taking all the necessary safety precautions, it is advisable to have tissue paper ready before opening the capillary connections.
To obtain the pressure value under normal operating conditions, multiply pressure values at lower flow rates by the factor: (normal flow rate)/(reduced flow rate).
If in doubt, use new capillaries or create a new connection by replacing fittings and ferrules. When using a cutting tool, ensure that the capillaries are not squeezed.
Case 2: Column Checks
If the system is functioning correctly and the columns are the root cause, they need to be tested one after the other.
Reinstall Columns and Sequentially Test:
Start by reinstalling the columns. Open the outlet capillary of the precolumn. Then proceed to open the outlet capillaries of the subsequent columns one by one to identify the specific column responsible for the pressure problems.
Very often the precolumn gets clogged over time and needs to be replaced, but analytical columns can also contribute to the pressure increase and may require replacement.
Investigate Pressure Changes After Sample Injection:
If the pressure increase occurs abruptly during a sample injection and remains elevated, further investigation is warranted. Consider sample incompatibility with the column stationary phase and review the sample chemistry, paying attention to functional groups in the sample that could cause problems. Consult the manufacturer’s information to explore suitable stationary phase materials or mobile phase additives required to successfully separate the sample by sizes only (3).
Summary
- Continuous pressure monitoring of a GPC/SEC system is extremely beneficial.
- The absolute value of the pressure depends on column characteristics, system, and method parameters. Temperature can also affect mobile phase viscosity and thus the overall pressure.
- Know your system and be familiar with the system pressure as well as the pressure with column installed. This helps to identify immediately whether columns or other LC system components are responsible for any unexpected behavior and allows for faster troubleshooting.
- An abrupt and sustained pressure increase with the injection of a sample may signal incompatible chemistries between the sample and stationary phase. Consider a more suitable stationary phase or mobile phase additives to analyze these samples.
References
(1) Held, D.; Radke, W. Tips & Tricks GPC/SEC: Flow Marker — An Easy Concept to Increase Reproducibility. Column 2016, 12 (6), 24–27.
(2) Held, D. Tips & Tricks GPC/SEC: The Art of Analyzing High Molar Mass Samples. Column 2014, 10 (10), 12–15.
(3) Reinhold, G.; Hofe, T. Tips & Tricks GPC/SEC: How to Find the Ideal Stationary Phase. Column 2007, 3 (12), 30–33.
Daniela Held studied polymer chemistry in Mainz, Germany, and works for PSS – A part of Agilent as an R&D director in Mainz. She is also responsible for education and customer training.
Michael Krämer studied polymer chemistry in Freiburg, Germany, and works for PSS – A part of Agilent as a solutions specialist. He is responsible for customer support and applications.
Email: WRadke@pss-polymer.com

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.
Influence of Concentration in Conventional GPC/SEC and Advanced Detection GPC/SEC
March 21st 2025Sample concentration is a parameter that can influence the quality of gel permeation chromatography/size-exclusion chromatography (GPC/SEC) separations and the obtained results. Understanding this influence can help to support the development of reliable GPC/SEC methods.