Market Profile: Dissolution Testing
Dissolution testing is a mandatory test for the physical evaluation of solid dosage forms such as capsules, tablets, ointments, and creams. The most basic form of testing measures the rate of dissolution or solubility of a drug tablet. Dissolution testing also can be used in ADME and bioavailability studies, release rates of a drug substance under different conditions, as well as provide information as to the efficacy of in-vivo performance.
Dissolution testing is a mandatory test for the physical evaluation of solid dosage forms such as capsules, tablets, ointments, and creams. The most basic form of testing measures the rate of dissolution or solubility of a drug tablet. Dissolution testing also can be used in ADME and bioavailability studies, release rates of a drug substance under different conditions, as well as provide information as to the efficacy of in-vivo performance.
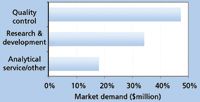
2005 dissolution testing demand by Industry
It is a common practice to link a dissolution tester with a high performance liquid chromatography (HPLC) system. This perhaps is one of the fastest growing areas in dissolution testing market. When the samples are introduced into the vessel, researchers remove small aliquots at regular intervals that will later be analyzed via high performance liquid chromatography (HPLC). Furthermore, fully automated online systems directly inject the sample into an HPLC and offer in-situ reaction monitoring capabilities.
Dissolution testing systems are used in a variety of laboratory settings including the R&D, quality control, and production steps of pharmaceutical development. The systems must be durable and provide quality information to meet regulatory requirements. In addition, because dissolution testing is so important to drug development, it is an essential tool for pharmaceutical companies.
In fact, the pharmaceutical industry accounts for an overwhelming majority of the market with strong growth from India and China fueling demand. But this industry is not the only consumer of dissolution testing instruments. Other industries like independent testing labs, CROs, and agriculture/food account for a respectable share of the market.
The foregoing data was extracted and adapted from Instrument Business Outlook, an SDi publication, and SDi's Global Assessment Report, 9th Edition. For more information, contact Glenn Cudiamat, VP of Research Services, Strategic Directions International, Inc., 6242 Westchester Parkway, Suite 100, Los Angeles, CA 90045, tel. (310) 641-4982, fax (310) 641-8851, e-mail: cudiamat@strategic-directions.com
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












