Managing Heterogeneity with Incremental Sampling Methodology
LCGC North America
Incremental sampling methodology laboratory processing is used to produce an appropriately sized subsample that has the analytes of interest at the same concentration as the large incremental sample collected in the field. The end goal is to produce results that represent the conditions at the site and facilitate good decisions.
Perhaps the largest source of error with sampling and sample preparation, especially with solid and semisolid samples, is the sample heterogeneity. Generally, sample heterogeneity is managed by sample homogenization, such as grinding and mixing, as well as use of an appropriately large sample size. Incremental sampling methodology (ISM) involves structured composite sampling and a processing method to create an unbiased estimate of the mean concentration of soil contaminants. Hence, ISM is emerging as a preferred methodology for conducting field environmental sampling. In this month's installment of "Sample Preparation Perspectives," we describe the application of ISM to laboratory subsampling protocols.
Incremental sampling methodology (ISM) is being used with increasing frequency to characterize heterogeneous environmental sites with chemical contamination. Site owners, government agencies, and consultants have significantly increased the use of ISM since guidance from the Interstate Technology and Regulatory Council (ITRC) was published in 2012 (1). ISM techniques are used both for initial sample collection in the field and for sample processing and subsampling at the laboratory. ISM laboratory processing is used to produce an appropriately sized subsample that has the analytes of interest at the same concentration as the large incremental sample collected in the field. The end goal is to produce results that represent the conditions at the site and facilitate good decisions. Reaching this goal requires that all sample handling steps contribute to achieving representativeness of the final subsample that is analyzed. Three different terms have been used to describe incremental sampling: multi-incremental sampling (MIS) (Envirostat Inc.), incremental sampling methodology (ISM), and incremental composite sampling (ICS) (2,3). Although there are some differences between these approaches, there is far more similarity.
Field sample collection involves taking many soil increments from a defined site area or volume, called a decision unit. Physically compositing these increments into a single sample then represents the average concentration of the chemicals of interest in the entire decision unit. An evenly spaced grid pattern, such as that shown in Figure 1, is the most common approach for increment collection. No additional processing of the composite sample is needed in the field. The sample is then shipped to the laboratory via same-day or overnight transport and, when needed for the analytes of interest, on ice.
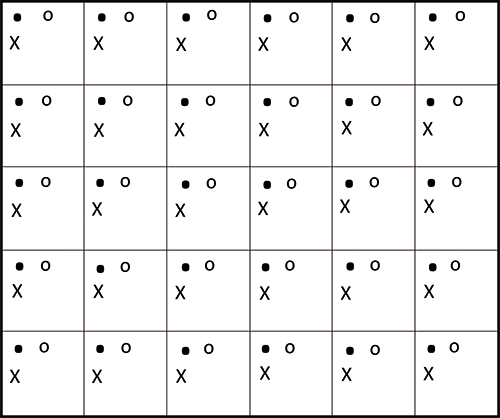
Figure 1: Systematic random sampling grid pattern used in ISM. Solid dots represent the primary sampling points. If replicate samples must be collected, "x" and "o" represent the duplicate and triplicate sampling points.
Typical laboratory processing options cover management of soil moisture, disaggregation, sieving, milling, sample splitting, and subsampling. Each of these laboratory processes has potential impacts on data quality and representativeness that should be considered when selecting the most appropriate options. These laboratory sample processing choices should be based on the specific project objectives to yield data that are usable for future site decisions. Let's take a closer look at each one.
Moisture Management
Most sample handling techniques are more easily applied to flowable soil samples. Thus, the most common first step is to air dry the sample at room temperature to reduce moisture to the extent that the soil clumps are easily crushed. Drying to constant weight (that is, no more moisture loss) is not necessary and prolongs the drying process, lengthens turn-around time, and increases the risk that lower-boiling semivolatile analytes will be partially lost. In some instances, weakly absorbed low-boiling analytes can be lost even with minimal air drying. Thus, direct subsampling of the as-received sample is necessary using the two-dimensional (2D) slabcake process (discussed later). In extreme cases, using volatile analyte soil processing techniques is the best choice. Thus, air drying is a tradeoff decision between ease of sample processing (that is, cost) and analyte representativeness. The specific project goals and objectives should be used to drive this decision.
Disaggregation
Breaking the dried soil clumps into free-flowing particles facilitates sieving, milling, and subsampling. Crushing these soil clumps can be accomplished by crunching in a mortar and pestle or in a small blender, such as that used for coffee bean milling. In either device, it is important to only break up the soil clumps and not mill the hard-crystalline particles into smaller particles.
Sieving
In some instances, it is appropriate to focus the analytical process on a specific size fraction of particles depending on the end use of the data. The use of a 2-mm sieve is a common operational distinction between soil and larger material. A 0.25-mm sieve has been used to assess the fraction of the sample that might stick to a child's hands and be accidentally ingested.
Milling
Complete particle-size reduction is performed with a mill, such as ball mill or puck (vibratory disk) mill. This complete particle size reduction improves subsampling reproducibility. It also releases the contents of the interior of the hard-crystalline particles for the measurement process. Thus, the measured concentrations of some elements can increase. Depending on how the data are used this increase might be considered a bias. Also, the mill materials and analytes of interest must also be considered to avoid contamination during the abrasive milling process. For example, a stainless steel mill should not be used if low levels of chromium are of interest, since chromium from the stainless steel is likely to abrade from the mill into the sample.
Subsampling
Even after all of the sample processing previously described, there can still be small-scale heterogeneity present. Thus, it is necessary to use a subsampling process that gives each particle in the sample an equal probability of being selected for the analytical subsample. For ISM samples, this is most commonly accomplished by spreading the powdered sample out in a large flat pan to an equal depth. This 2D slabcake is then divided into a grid. A random starting location in one grid cell, with replicates of that location in all the other cells, is selected, as shown in Figure 1. This is a small-scale version of what is frequently done in the field to collect the original sample. Each increment of sample should be collected with a square-end scoop with sides to avoid discrimination against the particles in either the top or bottom of the slab cake. The most common subsample sizes are in the 10–30-g range.

Figure 2: Air-dried soil samples showing "clumps" that can be readily broken apart.
The one-dimensional (1D) slabcake spreads the sample out in a long line. A large square scoop cuts perpendicular to the line and collects a large increment. Combining multiple large increments is useful when creating subsamples that are 100 g and larger. Custom disposable cardboard scoops are commercially available.
Larger Subsamples
Sampling statistics indicate that subsampling variability tends to be higher when the heterogeneous particles are larger. The fundamental error based on particle size may be estimated as follows:
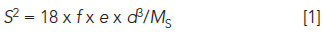
where S2 is the relative variance of the contaminant concentration because of the fundamental error, S is the relative standard deviation of the contaminant concentration because of the fundamental error, f is a dimensionless factor related to particle shape (0.5 can be considered a typical value [4]), e is the average density (g/cm3) of the population (2.5 g/cm3 is considered an average value), d is the diameter, in centimeters, of the largest particle, and MS is the sample mass in grams (5). This fundamental error is related to the average precision one should expect for a given subsample size. Gy's sampling theory (4,6–8) provides a thorough treatment of the types and magnitude of error associated with sampling.

Figure 3: Example of ball mill in use for reducing the size of soil particles.
Figure 4 illustrates that subsampling precision is directly correlated with both sample size and particle size. This figure demonstrates one of the key benefits of sample milling. Smaller particles tend to produce more reproducible results. In some scenarios, milling is not desirable or available. In these instances, increasing the subsample size that is taken through sample digestion or extraction steps can also help to manage precision. Thus, many laboratories that provide environmental ISM services have also increased subsample masses from 1 to 10 g for metals digestions via Environmental Protection Agency (EPA) Method 3050 (9). This increase in subsample size and the ISM processing options discussed above will be incorporated into future EPA methods.
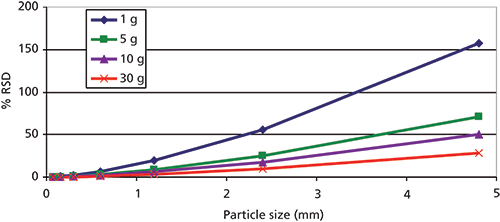
Figure 4: Fundamental error plotted as a function of particle size and sample size. Error decreases as particle size decreases or sample size increases.
Equipment Blanks
Monitoring the sample handling processes for contamination either from equipment materials or carryover from previous samples is desirable, but not simple. There is not a single soil-like matrix that is completely clean with respect to all possible environmental semivolatile and nonvolatile analytes. Clean sand can be used as a blank matrix for many organic analytes. However, there is no soil-like solid matrix that is below the detection limit for all metals of environmental interest. The most common approach is to rinse the equipment with reagent water to test for soil particle carryover from previous samples. This is similar to how field sampling equipment is tested after decontamination.
Discussion
Although air drying, disaggregation, sieving, and 2D slabcake subsampling is the most commonly selected option for ISM sample processing, there is no "one size fits all" option that is the best choice for every site characterization project. There are two fundamental decisions and two trade-off decisions to be made by the project team.
The first fundamental question is: What are you looking for? Knowing the analytes of interest and their properties will lead toward certain processing options. The second fundamental question is: Where are you looking? This is not where on the site, but rather where in the sample container. Some project objectives call for removing large organic materials, rocks, or all particles >2 mm. Also, is it important to look only at the exposed surfaces and pores of the sample particles or does the sample process need to expose the interior of the particles?
The first trade-off question relates to air drying the field samples. Air drying converts the soil sample into a dry form that is easier and less expensive to process. However, low-boiling analytes can be lost during drying if they are not strongly absorbed to the sample particles. The second trade-off decision relates to milling. Milling produces smaller particles, which leads to more reproducible subsampling and hence better precision in the final results. However, milling also releases metals that are trapped inside the particles into the measurement process. Also, many mills are made of metallic components, which tends to increase the measured concentrations for selected metals.
ISM has been applied to different sites and analytes. Example sites include military training ranges, urban gardens, underground storage tanks, parks, residential neighborhoods, and skeet shooting ranges. Analytes include explosives, metals, polycyclic aromatic hydrocarbons (PAHs) and other semivolatile organics, petroleum hydrocarbons, dioxins, organochlorine pesticides, and polychlorinated biphenyls (PCBs). ISM principles can be applied to volatile organics using an adaptation of EPA Method 5035 using methanol extraction.
Conclusion
To recap, ISM laboratory processing is used to produce an appropriately sized subsample that has the analytes of interest at the same concentration as the large incremental sample collected in the field. The end goal is to produce results that represent the conditions at the site and facilitate good decisions. ISM processing principles were originally developed for mining and agricultural applications before being adapted for environmental applications. The same principles are applicable to other heterogeneous sample types.
References
(2) www.envirostat.org.
(3) www.epa.gov/superfund/site-evaluation-dioxin-superfund-sites.
(4) P.M. Gy, Sampling of Particulate Material (Elsevier, Amsterdam, The Netherlands, 1982).
(5) C.A. Ramsey, M. Ketterer, and J.H. Lowry, "Application of Gy's Sampling Theory to the Sampling of Solid Waste Materials" in Proceedings of the EPA Fifth Annual Waste Testing and Quality Assurance Symposium 11, 11–494, (Washington, DC, 1989).
(6) P. Gy, Sampling for Analytical Purposes (Wiley, New York, New York, 1998).
(7) F.F. Pitard, Pierre Gy's Sampling Theory and Sampling Practice, Heterogeneity, Sampling Correctness, and Statistical Process Control, 2nd Edition (CRC Press, Boca Raton, Florida, 1993).
(8) R.W. Gerlach, J.M. Nocerino, C.A. Ramsey, and B.C. Venner, Anal. Chim. Acta 490, 159–168 (2003).
(9) K. Edgell, US EPA Method Study 37 - SW-846 Method 3050 Acid Digestion of Sediments, Sludges, and Soils. (U.S. Environmental Protection Agency, Washington, D.C., EPA Contract No. 68-03-3254, 1988).
ABOUT THE AUTHORS
Mark Bruce

Mark Bruce is the Corporate Technical Director at TestAmerica in North Canton, Ohio. He has more than 35 years of environmental analytical chemistry experience in academic and commercial laboratories. He has led method development and modification projects for both organic and inorganic analyses, which include arsenic speciation, methylmercury, low-level mercury, vapor space organics, leaching, and incremental sampling methodology (ISM). Dr. Bruce has been actively involved with the Interstate Technology and Regulatory Council (ITRC) committee for ISM, and is a recipient of the ITRC Industry Recognition Award for his contribution.
Douglas E. Raynie

Douglas E. Raynie "Sample Prep Perspectives" editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@ubm.com







