Man of the Masses
MS: The Practical Art Editor Kate Yu interviews Fred McLafferty about his pioneering career in mass spectrometry
"MS: The Practical Art" Editor, Kate Yu, spoke to Fred McLafferty about his pioneering career in mass spectrometry (MS).
Kate Yu: What brought you into the field of mass spectrometry (MS)?
Fred McLafferty: A crazy coincidence! After a PhD at Cornell University, (Ithaca, New York, USA) and a postdoc at the University of Iowa, (Iowa, USA), I arrived at the Dow Chemical Co., (Midland, Michigan, USA), in 1950 for an interview in their organic chemistry research laboratory. However, Dow also interviewed me for their spectroscopy laboratory in the MS group of Vic Caldecourt and two instrument operators. Vic had made the MS analyses so popular within the company that Dow wanted a chemist for the increased sample load while he concentrated on maintaining and improving the cranky instrumentation, at which he was terrific. No one, not even me, understood why I took the job with absolutely no prior knowledge (1).

Bendix time-of-flight mass spectrometer at the Dow Chemical Company with Roland Gohlke (foreground) and Fred McLafferty. Published with permission of Fred McLafferty.
KY: You were in World War II and were awarded the Combat Infantryman Badge. What were the most remarkable experiences you remember during the war?
FM: I went on active duty in April 1943, just after finishing a BS in chemistry and mathematics at the University of Nebraska (Nebraska, USA). A month before the war ended in Europe, our 2nd Battalion, 253rd Infantry, captured a major remaining German ammunition depot in a fierce battle against an elite SS unit that promised Hitler "no retreat". The battalion members were awarded a Presidential Unit Citation. During this action platoon sergeant John R. Crews of my rifle company was awarded the Congressional Medal of Honor for personally saving part of another company that was trapped. I was wounded earlier the same day.
KY: Why did you come back to the field of chemistry after the war?
FM: I was offered a battlefield commission from sergeant to officer rank, so I had a "career choice", but I doubt that such a career would allow the "fun participation" that I am having at my present age.
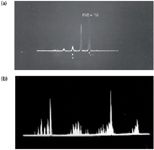
Time-of-flight mass spectra (oscillograph display) typical of Dow’s early GCâMS capabilities. (a): Mass spectrum of methane (CH4), peaks at m/z = 12â17 (16 tallest). (b): Mass spectrum of acetone (C3H6O, MW 58), peaks at 13â59 (contains impurity peaks, for example, H2O). Published with permission of Fred McLafferty.
KY: Looking back at your career, who was the most influential person for you?
FM: Professor Franklin A. Long, who was at the Cornell University Chemistry Department from 1937 to 1999, was a very special scientific role model and friend during my graduate work from 1947 to 1949, and after I joined the faculty in 1968. He was a member of the National Academy of Sciences, Assistant Director of the US Arms Control and Disarmament Agency, and on the Science Advisory Committees for three US Presidents. But by far the most important person in my life for 65 years has been my wonderful wife: Elizabeth "Tibby" Curley McLafferty.
KY: Tell us about your experiences at Dow Chemical. How did you develop the GC–MS instrument?
FM: Dow's spectroscopy laboratory was one of the best in "modern" high information methods including direct reading atomic emission spectroscopy (AES), X-ray diffraction (XRD), infrared (IR), nuclear magnetic resonance spectroscopy (NMR) and MS. Dow was also unusually supportive of any new MS applications and related research. At a 1954 Gordon Research Conference, friend Steve DalNogare of duPont and H.N. Wilson from Imperial Chemistry Industries (ICI) (Manchester, UK), told me about gas chromatography (GC) with full details on how to build one. Soon after, Roland Gohlke, who worked with me, developed an improved version to solve problems in Dow laboratories and plants. For example, Roland put the column, a 25-ft copper tubing coil, in a 2L Dewar for better temperature control and often used the detergent "Tide" as a column packing.

Fred McLafferty (far left); Roman Zubarev, Professor of Medical Proteomics in the Department of Medical Biochemistry and Biophysics at the Karolinska Institutet (Stockholm, Sweden). Roman also directs the institute’s large LCâMS proteomics facility; Dr Gary Valaskovic, co-founder and CEO of New Objective (Woburn, Massachusetts, USA); Neil Kelleher, Professor in Chemistry, Molecular Biosciences and the Feinberg School of Medicine of Northwestern University (Chicago, Illinois, USA). Neil also directs the large LCâMS proteomics facility in Chicago. The photo was taken by Dr Susan Weintraub at the ASMS meeting (Philadelphia, USA) June 2009. Published with permission of Fred McLafferty.
In addition, my friends Bill Wiley and Dan Harrington at nearby Bendix Research Labs were just commercializing their time-of-flight (TOF) MS instrument (10K spectra/s), and were happy to let us try coupling our GC to the TOF in February 1956. It was very exciting to see on the output oscilloscope the rise and fall of unit-resolution spectra of acetone, benzene and toluene, the first organic compounds run on their TOF instrument (2).
KY: How did you discover the famous McLafferty Rearrangement and what sort of impact did it have on mass spectrometry?
FM: The infrared capabilities of Dow's spectroscopy laboratory were world-class, helped by the spectroscopy team's extensive efforts to collect a large spectral database of organic compounds. Consequently, during any spare MS instrument time we had, we continued this to build up an MS spectral database using the extensive sample collection available, giving us the mass spectra of many compound classes. This made it possible by 1956 to show (3) a general correlation of novel hydrogen rearrangements, although individual cases had previously been reported.

At that time Dow sent me to the Boston area to set up a corporate laboratory for basic research. Although for my personal research I had no MS instrument, the structural diversity of our MS database was already unique, allowing extensive details of single and double hydrogen atom rearrangements to be established (4). This helped to show the key role of odd-electron ions in MS ion dissociation mechanisms and convince organic chemists that mass spectra were based on real chemistry.
KY: Why did you go back to university while having a fantastic career in industry at the time?
FM: The success of Dow's new basic research laboratory depended on selling its research capabilities to the academic community, as well as to meet Dow objectives. New hires found a novel research opportunity with industrial level support. We did recruit great people, including later Nobel Laureate George Olah. But in trying to sell my industrial research to academe, they sold me on academia. In 1964 I went to Purdue University (West Lafayette, USA), as Professor of Chemistry.

KY: You've made many great contributions to mass spectrometry over the years. What do you think is your greatest contribution?
FM: In 1950 MS was dominated by measurements of isotope ratios, atomic weights and hydrocarbon mixtures, while my main research efforts since then have been in "molecular mass spectrometry". The current reversal in dominance came through a long series of developments that our research contributed to. These included an improved understanding of new ion/radical chemistry; advances in GC–MS, LC–MS, MS–MS instrumentation; advances in techniques, such as collisional activated dissociation, neutralization–reionization and electron capture dissociation; improved computer data acquisition, reduction and identification using collected reference data (5); and developments in top-down characterization of biomolecules and gaseous protein conformers (1). It was the combined efforts of the unusually cooperative researchers of many MS laboratories that made these advances possible.
KY: Among all the awards you have received, which one was the most remarkable award to you?
FM: Al Bard and I were elected to the US National Academy of Sciences in 1982; before that, the only analytical chemists elected were I.M. "Pete" Kolthoff (1958) and Charlie Reilley (1977). Best of all, I have also been awarded a most remarkable family of five children and ten grandchildren.
KY: What major challenges remain in mass spectrometry? Which ones do you think the younger generation should focus on?
FM: The huge growth of MS removes its applications further from the basic research that makes more of this growth possible. Education awareness requires updated textbooks, "short courses", consultants and, most importantly, improved communication in our field. The positions of those supporting "bottom-up" versus "top-down" approaches appear to have shifted little in the last decade.
KY: Where do you think the future of mass spectrometry lies?
FM: As in 1950, I believe that the future is still in "molecular mass spectrometry". But now we see more clearly the amazing enhancements possible with far greater mass range (megadalton+) and resolving power, coupled separation techniques (for example, MS with chromatography, ion mobility) and computerized automation. These can be applied to an even broader range of critical areas of basic knowledge and practical problems.
KY: Is there any advice you would give to young scientists embarking on a career in analytical chemistry?
FM: Young scientists should please note that analytical chemistry overall has a great future. In 1984 I proposed the Analytical Criteria of "6 Ss": Specificity, Sensitivity, Speed, Sampling, Simplicity and $. Improve one or more of these in an old method, or find a new method with potential in these criteria — solving problems has great rewards!
KY: Do you have hobbies outside your work?
FM: "Hobby" is not a fair descriptor, but I am lucky to spend most of my time outside MS with my extended family.
References
(1) F.W. McLafferty, Annu. Rev. Anal. Chem. 4, 1–22 (2011).
(2) R.S. Gohlke and F.W. McLafferty, J. Am. Soc. Mass Spectrom. 4, 367–371 (1993).
(3) F.W. McLafferty, Anal. Chem. 28, 306–316 (1956).
(4) F.W. McLafferty, Anal. Chem. 31, 82–87 (1959).
(5) F.W. McLafferty, Registry of Mass Spectral Data (and Registry combined with NIST), 9th Ed., Wiley-Blackwell: Hoboken, N.J., USA, (2009).
(6) F.W. McLafferty, Science 226, 251–253 (1984).

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).












