Making HILIC Work for You—Column Selection
Tips on how to select a hydrophilic interaction chromatography (HILIC) stationary phase.
Tips on how to select a hydrophilic interaction chromatography (HILIC) stationary phase.
Hydrophilic interaction chromatography (HILIC) requires a hydrophilic stationary phase to adsorb a water layer that allows the analyte partitioning process to take place. The hydrophilicity of the column packing material influences the thickness of the water layer, which directly impacts analyte retention. Other interactions between the analyte and stationary phase, such as electrostatic and hydrogen bond interactions, can also be manipulated by selecting stationary phase chemistries that contain polar or charged functional groups.
Modern HILIC stationary phases are either bare silica or polar chemically bonded phases that contain ionizable ligands bonded to the silica surface. The use of ligands capable of undergoing electrostatic interactions can add an extra dimension to the separation when analyzing ionizable compounds. HILIC stationary phases can be divided into three main categories:
- Neutral: Polar surface with no electrostatic interactions.
- Charged: Strong electrostatic interactions from anionic or cationic functional groups.
- Zwitterionic: Weak electrostatic interaction; the stationary phase contains both cationic and anionic functional groups.
Bare silica phases can be used for the separation of small polar compounds, such as carbohydrates, peptides, and proteins. Silica surfaces contain acidic surface silanol groups that have a pKa of 3.5, this means that above pH values equal to the pKa these groups will become ionized, allowing the silica stationary phase to work as a cation exchanger that can interact with and retain positively charged basic analytes.
Neutral HILIC phases predominantly comprise polar functional groups-for example, amides, aspartamide, diol, cross-linked diol, cyano, and cyclodextrin. These groups are in their neutral form in the pH ranges typically used for HILIC mobile phases (pH 3–8). The retention mechanism is via hydrophilic interactions. Neutral HILIC phases have been applied to the separation of monosaccharides, peptides, amino acids, and small polar molecules.
The most common functional group used in charged phases is an aminopropyl group. The primary amino group is positively charged; therefore, it exhibits high affinity for anionic acid compounds-that is, amino acids, peptides, antibiotics, carboxylic acids, and nucleosides. The retention mechanism is based on anion exchange. These stationary phases are highly hydrophilic; therefore, they are amenable to the retention and separation of neutral polar compounds.
Zwitterionic HILIC phases are the most versatile and are often the first choice of column during method development. Zwitterionic phases contain functional groups that are permanently positively and negatively charged-for example, quaternary ammonium (positive) and sulfonic acid (negative) functional groups. The hydrophilic nature and weak ionâexchange properties allow the separation of neutral, acidic, and basic analytes, polar and hydrophilic compounds, and inorganic ions.
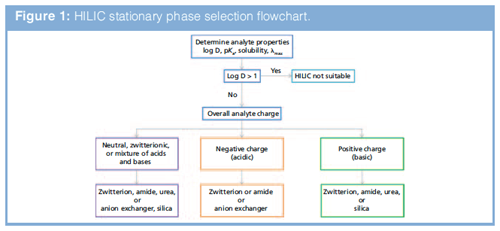
Stationary phase selection is based on the chemical properties of both the column and analytes. HILIC is designed for use with analytes that have a log D < 0, (the more negative the log D value for an analyte, the greater the degree of stationary-phase polarity required to retain it). Log P, pKa, and solubility also need to be considered when designing methods. Figure 1 shows a flow chart for selecting an appropriate HILIC stationary phase. Phases that have little ion-exchange activity are suitable for the separation of neutral zwitterionic and acid–base mixtures. Phases that have considerable ion-exchange activity are suitable for analyzing acids (anion-exchange phases) and bases (cation-exchange phases). A zwitterionic stationary phase is often a very good starting point. If there is not enough information about the analytes, screening several stationary phases is recommended. For standard HILIC applications use a 100- or 150-mm column with 3–5 µm particles and a 4.6-mm internal diameter when using ultraviolet detection. For applications that use mass spectrometry or charged aerosol detection, smaller columns, such as 100 mm × 2.1 mm or 50 mm × 2.1 mm, 3–5 µm dp, are optimum (reducing column length will decrease the number of analytes that can be separated because of decreased efficiency). Subâ2âµm fully porous or superficially porous materials can increase efficiency and resolution.
Find this, and other webcasts, at www.CHROMacademy.com/Essentials (free until 20 May).

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.











