Living Off the Fat of the Land
LCGC North America
Recently, Nature and Science Citation Index listed the 100 most cited research papers of all time. Two of these are the classic Bligh-Dyer and Folch lipid extraction methods from the late 1950s. This month we will take a look at the lasting impact of these papers and explore the current state of lipid extractions, including lipidomics.
Recently, Nature and Science Citation Index listed the 100 most cited research papers of all time. Two of these are the classic Bligh-Dyer and Folch lipid extraction methods from the late 1950s. This month we take a look at the lasting impact of these publications and explore the current state of lipid extractions, including lipidomics.
In October, Nature published a list of the 100 most cited articles of all time, based on Science Citation Index (1,2). I perused the list expecting to find classic articles like Watson and Crick’s description of the structure of DNA (not in the top 100, with only about 5200 citations), Kary Mullis’s development of polymerase chain reactions (PCR) for DNA amplification (number 63 on the list), or, as an analytical chemist, some of Martin and Synge’s pioneering chromatography work (also, not on the list). One hypothesis claimed by the study authors is that these classic works rapidly become so familiar that they do not require citation.
Despite the dearth of some of these groundbreaking works, I was heartened to see that two extraction articles, the Folch (3) and Bligh–Dyer (4) fat extraction methods, were listed in the top 20 most cited articles. The Folch article received 45,131 citations at the time of the study, while the Bligh–Dyer article was cited 32,131 times. One might expect that the citation frequency would follow a somewhat normal distribution with an increasing number of citations shortly after publication and as the immediate impact is realized, then taper off as the research becomes widely accepted or replaced with further investigations. Yet, both of these lipid extraction methods are still receiving an increasing number of citations, each approaching 1500 citations per year. Lipid extractions are commonplace, for example, for nutritional studies and compliance with the Nutritional Labeling and Education Act, recovery of oils from oilseeds, or the emerging field of lipidomics. But given that these articles are approaching 60 years since publication, let’s take a look at them and the field of lipid extraction. One expert in the field postulates that these methods are often widely misunderstood and that few of those that cite these papers have actually read them (5); it is hard to disagree with this assessment.
As we begin a discussion of lipid extractions, we must understand the diverse nature of lipids. Most of us are familiar with the simple lipids, the predominately nonpolar mono-, di-, and triglycerides and free fatty acids that make up the bulk of dietary fats. These are typically found in adipose tissue, oilseeds, and similar tissues. In many studies, knowledge of the simple lipids is sufficient and extraction with nonpolar solvents can be quantitative. Complex lipids are the minor polar lipid classes like phospholipids, gangliosides, sphingolipids, and inositides that serve important biological roles such as cellular signal transduction and indicators of certain health or disease states.
The Folch and Bligh–Dyer Methods
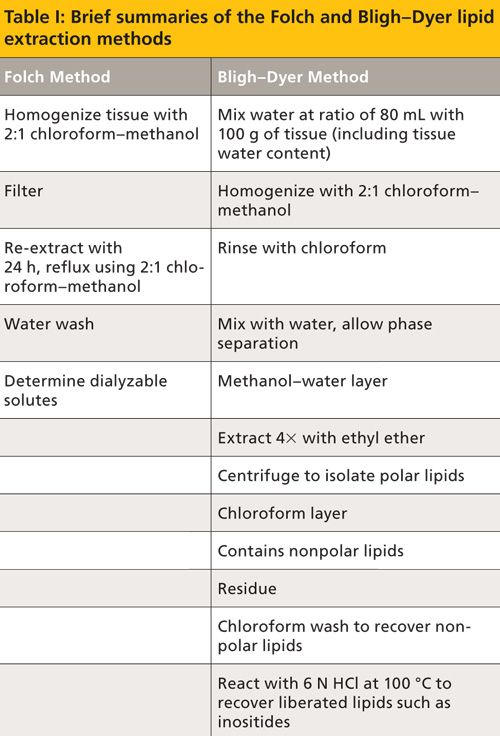
The Folch and Bligh–Dyer methods are summarized in Table I. It should be noted, however, that a full understanding of the methods and their applications cannot be gleaned by such a tabulation. These methods introduce 2:1 chloroform–methanol as a solvent system for the extraction of total lipids. Importantly, according to the Folch method, the ratio of solvents must remain as close as possible to 8:4:3 (the tertiary solvent being endogenous water in the tissue sample). The Folch method predates the Bligh–Dyer method and includes a 24-h reflux step. Hence, the Bligh–Dyer method was developed as a less time- and solvent-intensive method, especially for tissues like fish, which possess low lipid levels (below about 2%) and high water content. This improved method also states that, “due to the highly unsaturated nature of fish lipids, the method had to involve only mild treatments so as to minimize oxidative decomposition and the production of artifacts” (4). Success of the Bligh–Dyer method depended on keen attention to the phase behavior of the chloroform–methanol–water system. Christie (6) notes that the capacity of the chloroform to form weak hydrogen bonds with water leads to the success of the chloroform–methanol system. However, health and safety concerns with chloroform have led to a plethora of investigations using solvents like hexane–isopropanol or ethyl acetate–ethanol.
Considerations for Other Lipid Extractions
In addition to lipid polarity, considerations like the attraction between the lipids and cellular proteins, polysaccharides, and other materials must be taken into account. The polarity differences between the hydrophobic hydrocarbon chain and the more polar end groups must be balanced. The Christie review summarizes potential lipid extraction solvents (6): triglycerides, cholesterol esters, and other nonpolar lipids can be extracted with hydrocarbons like hexane, toluene, cyclohexane, or the slightly more polar diethyl ether. These lipids are soluble in alcohols with increasing chain length. Lipids with fatty acid moieties require increasingly polar solvents for extraction. High dielectric lipids require the most polar solvents such as methanol, ethanol, or even water. Diethyl ether is frequently used by regulatory agencies and trade organizations for the determination of fat in foods. But this solvent also coextracts nonsaponifiable, non-nutritive lipids, while being insufficient for complete extraction of dietary lipids (7). Meanwhile Thiex, Anderson, and Gildemeister compared the Randall, Soxhlet, and submersion methods for lipid extraction using diethyl ether, petroleum ether, hexanes, and pentanes (8). They found that while hexanes generally only extract about 97% of the lipid matter compared with diethyl ether, the submersion method was less time and labor demanding, allowing hexanes to become an acceptable alternative to diethyl ether. Christie also describes means to minimize the formation of lipid degradation products via transesterification, autoxidation, and other mechanisms (6).
More recently, matrix solid-phase dispersion was developed as a means of simultaneously disrupting solid samples and extracting contaminants from lipid matter (9). A mortar is used to grind bonded-phase sorbents with tissue samples to isolate drugs, pesticides, or pollutants from tissue samples. This dispersive solid-phase extraction approach also provides the basis for the QuEChERS (quick, easy, cheap, effective, rugged, safe) technique (10).
Lipidomics
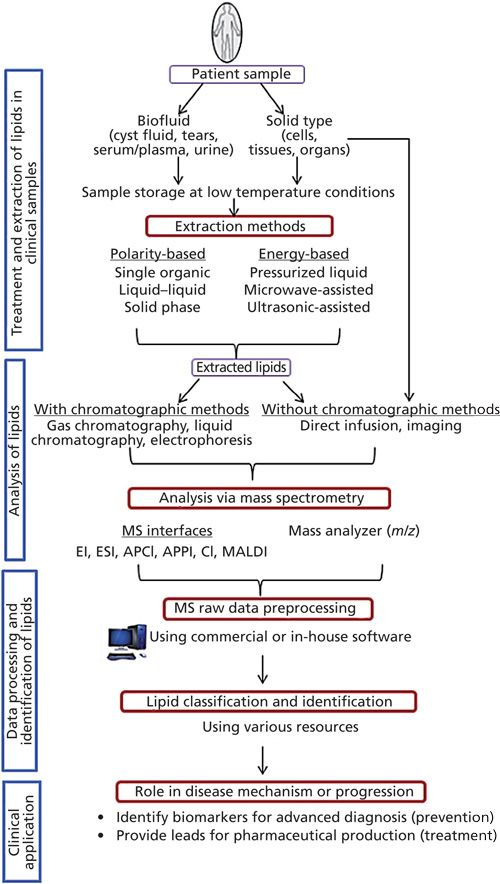
The study of complex biochemical processes via the lipidomics approach presents several additional challenges. Not only are the polar, minor lipids subject to scrutiny, the tissue samples under study are often limited to tens or hundreds of milligrams. In addition to the chloroform:methanol extraction solvent, 80% methanol and methyl tert-butyl ether (MTBE) are also used (11), with the advantage that MTBE leads to more clean phase separation. Lipidomics commonly uses single organic solvent extraction (SOSE), typically with methanol or acetonitrile, or liquid–liquid extraction (LLE) with chloroform-methanol (12). A recent review provides a comprehensive look at the sample preparation and analysis considerations in lipidomics (12). This review includes a comprehensive table compiling the advantages and disadvantages of SOSE, LLE, solid-phase extraction, solid-phase microextraction, supercritical fluid extraction, microwave-assisted extraction, and ultrasound-assisted extraction. For these investigations, it appears simplicity of extraction is preferred because of the resolving power of the mass spectrometric methods used. A workflow for clinical lipidomics is presented in Figure 1.
Conclusion
Lipid extractions take on many forms because of the complexity and concentration of the lipids to be studied. For this reason, the classic lipid extraction methods by Folch and Bligh–Dyer are not only still valid, they must be understood as worthy methods for routine laboratory use and to provide a springboard to the development of new techniques.
References
(1) R. Van Noorden, B. Maher, and R. Nuzzo, Nature514, 550–553 (2014).
(2) www.nature.com/top100.
(3) J. Folch, M. Lees, and G.H. Sloane Stanley, J. Biol. Chem.226, 497–509 (1957).
(4) E.G. Bligh and W.J. Dyer, Can. J. Biochem. Physiol.37, 911–917 (1959).
(5) http://lipidlibrary.aocs.org/topics/archive/extraction.htm.
(6) W.W. Christie, Advances in Lipid Methodology - Two (Oily Press, Dundee, UK, 1993), pp. 195–213.
(7) D.L. Palmquist and T.C. Jenkins, J. Animal Sci.81, 3250–3254 (2003).
(8) N.J. Thiex, S. Anderson, and B. Gildermeister, JAOAC Intl.86, 888–908 (2003).
(9) S.A. Barker, A.R. Long, and C.R. Short, J. Chromatogr. A475, 353–361 (1989).
(10) M. Anastassiades, S.J. Lehotay, D. Stajnbaher, and F.J. Schenck, JAOAC Intl.86, 412–431 (2003).
(11) S. Chen, M. Hoene, J. Li, Y. Li, X. Zhao, H.-U. Haring, E.D. Schleicher, C. Weigert, G. Xu, and R. Lehmann, J. Chromatogr. A1298, 9-16 (2013).
(12) C.C. Teo, W.P.K. Chong, E. Tan, N.B. Basri, Z.J. Low, and Y.S. Ho, TrAC Trend Anal. Chem.66, 1-18 (2015).

Douglas E. Raynie
“Sample Prep Perspectives” editor Douglas E. Raynie is an Associate Research Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







