The LCGC Blog: Silica for HPLC Stationary Phases – A Five Minute Guide
The nature of surface silanol groups is very important to determine the selectivity of a stationary phase. This article illustrates what analysts should be looking at on the silanol surface to asess the separation mechanism.
kseniasol/stock.adobe.com

The nature of surface silanol groups is very important to determine the selectivity of a stationary phase. This article illustrates what analysts should be looking at on the silanol surface to asess the separation mechanism.
Most high performance liquid chromatography (HPLC) columns are packed with silica onto which some form of hydrophobic ligand is bonded. These columns form the vast majority of those used for modern reversed-phase HPLC.
The chemical and physical characteristics of the silica may, in some instances, have just as big an impact on the selectivity of the separation as the bonded phase ligand, and we need to be aware of the effect of the silica stationary-phase support on our separations and what to do when things are not going as we might expect.
Surface Area and Pore Volume
HPLC separations require a large surface area of hydrophobic material to act as the stationary phase for the separation, and there is a direct correlation between available surface area of the packed bed and column efficiency (or plate count, which governs the width per unit time of each chromatographic peak). Most modern HPLC packing materials of 5-mm particle diameter or lower will have a surface area of between 150 and 400 m²/g. Just for comparison purposes, the surface area of an average tennis court is around 260 m², while a 100 mm × 4.6 mm HPLC column will contain around 1g of silica and will therefore have a surface area somewhere between half and double the size of the tennis court! However, surface area alone is not a good measure of column retentivity, which will also depend upon the pore volume of the silica used. Manufacturers may use measurements of surface area versus pore volume to assess the true retentivity of the phase, which will be further adjusted to take into consideration the carbon loading (%C), a measure of the amount of bonded phase ligand per unit volume or weight of silica. Columns with large surface area, large pore volume, and high carbon content will be highly retentive towards hydrophobic analytes, and vice versa. Furthermore, the correlation between available surface area and carbon loading will give an indication of the spacing of the ligands on the silica surface, and therefore how “accessible” the surface is to our analyte – which may have a pronounced effect on the selectivity of the separation.
Particle Size Distribution
The silica particle size will determine the permeability of the packed bed as well as the efficiency of the column. Smaller particles generate higher efficiency as well as higher back pressure. All particles used for modern HPLC come from a distribution and the wider the distribution, the lower the efficiency of the packed bed. A popular theory as to the high efficiency of core-shell type columns relates to the efficiency of the packed bed which results from the core-shell particles naturally forming a markedly lower distribution of particle sizes due to the fixed diameter of the solid cores used.
System back pressure (column permeability) is inversely related to the square of the particle diameter – and therefore a 5% shift in average particle diameter will result in a 10% change in column back pressure. A manufacturer’s ability to produce silica particles from very similar particle size distributions from batch to batch is important to us to ensure a consistent column efficiency and system back pressure for each column we purchase. Particle size distributions of <<25% around the mean particle size are to be expected for modern materials.
Surface Chemistry
Figure 1 shows a silica surface containing important elements in reversed-phase chromatography – of course this is a contrived diagram and no surface would be expected to contain all of these elements in such close proximity.
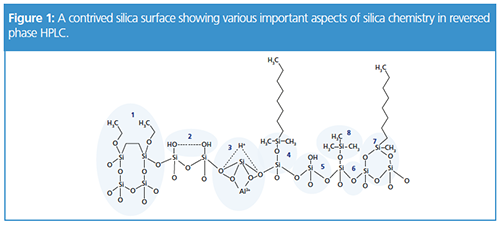
Element 4 in Figure 1 shows a typical bonded phase ligand on the silica surface. A C8 dimethyl chlorosilane ligand has been attached via a condensation reaction to a single surface silanol species – this is typical of the mechanism by which many HPLC stationary phases are manufactured. The ligand is bound via a siloxane bridge (Si-O-Si), and it is this species that is susceptible to hydrolytic attack when working at low eluent pH – meaning the phase is stripped from the silica surface. Under these circumstances, a gradual loss of retention or efficiency will be noted alongside increased peak tailing of polar and ionized species. Manufacturers may use bulky substituents (isobutyl or isopropyl) to replace the two methyl moieties in order to sterically protect the siloxane bond from hydrolytic attack. Interestingly, due to steric factors (the ligand will not of course be at 90° to the silica surface as shown in Figure 1!), typical surface coverage of the bonded phase will be 40–50% of the total available silanol sites (typically 8 mmol/m²).
Element 7 shows a “bi-functional” ligand, that is typically formed when di-chlorosilane species are used as the bonded-phase ligand. This is thought to increase the hydrolytic stability of the bonded phase, although this approach runs the risk of any non-bonded chlorosilane moiteties being hydrolysed to form hydroxyl species that may contribute to peak tailing with polar and ionisable analytes. Some manufacturers claim to use tri-functional bonding, however there is a significant body of literature that indicates that true tri-functional bonding is not possible with the type of silica used for HPLC stationary phase manufacture.
In order to “mask” the remaining silanol groups (which can, under certain circumstances, be detrimental to analysis), manufacturers use “end-capping” reagents. These are typically silanols of the type used for the bonded phase ligand, with short alkyl chains or a methyl group to reduce their steric profile. A trimethyl silyl end-capping reagent is shown in Element 8 (Figure 1). Even using aggressive bonding conditions and highly reactive reagents, a “fully endcapped” silica surface rarely contains less than half of the original silanol species!
The nature of surface silanol groups is all-important in determining the selectivity of a stationary phase. While steric factors may preclude surface access to a bonded phase ligand, our analyte may have access to the surface silanol species which remain in the non-derivatised state. There are several conformations of silanol group, however Elements 2 and 5 in Figure 1 show two of the most important. In Element 5, a lone or acidic silanol is shown. This type of silanol is highly surface active and, with a pKa of around 3.8–4.2, depending upon eluent pH, the group will be capable of interacting with polar or ionisable analyte functional groups, resulting in analyte peak tailing. At increased pH (> 6) the silanol group will be fully ionised and will interact strongly with protonated basic species. At low pH (< 4), then the degree of interaction with basic species will be much less as the silanol will be protonated – a primary reason behind the proliferation of methods at low pH in the pharmaceutical industry, in which many of the molecules historically involved basic moieties.
Element 2 shows an approach used by many manufacturers to overcome this situation. It should be borne in mind that very often, while the overriding retention mechanism is hydrophobic in nature, the influence of the interaction between polar or ionisable analyte functional groups and polar moieties on the silica surface can lend the selectivity required to achieve a separation. Processing of the silica (typically at high temperature) can lead to the formation of swathes of silanol groups which are in the vicinal (inter-hydrated) form as shown in Element 2. These silanol groups are capable of interacting with the analyte polar functional groups but are of low enough energy not to give rise to significant peak tailing. This type of silica is known as Type B or Type II and can add a polar influence to a reversed-phase separation which can assist with the separation on analytes whose basic scaffold is similar but where there are differences in functional group chemistry.
Element 3 shows yet another potentially harmful silica surface phenomenon. The inclusion of metal ions within the silica matrix (especially aluminium, sodium and iron) may lead to the association of protons onto the silica surface via co-ordination effects. The presence of the protons leads to a highly acidic surface environment and these species will readily interact with acidic analyte functional groups to cause peak tailing. Most manufacturers will use various techniques to wash the silica in order to remove the metal ions – however this may ultimately lead to a silica which is mechanically less stable and may crush more easily under high pressure operating conditions, leading to column voids and broad and/or shouldered peaks.
When using high pH eluents, the silica matrix itself will be subject to hydrolysis (Element 6), which will ultimately cause the silica particles to dissolve, causing a column void and a gross reduction in efficiency with tailing or shouldered peaks, usually combined with a gradual increase in system back pressure.
Element 1 shows a silica chemistry which uses alkyl bridges to present far fewer surface silanol groups and which is therefore much less susceptible to acid or base hydrolysis and these ‘hybrid’ silica materials show a greater resistance to pH and can be used outside the traditional silica working pH range of 2.5–7.5.
Tony Taylor is Group Technical Director of Crawford Scientific Group and CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting, working with Crawford Scientific Group clients to ensure they attain the very best analytical science possible. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science. His current research interests include HPLC column selectivity codification, advanced automated sample preparation, and LC–MS and GC–MS for materials characterization.
E-mail:tony@crawfordscientific.comWebsite: www.chromatographyonline.com

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
New Study Investigates Optimizing Extra-Column Band Broadening in Micro-flow Capillary LC
March 12th 2025Shimadzu Corporation and Vrije Universiteit Brussel researchers recently investigated how extra-column band broadening (ECBB) can be optimized in micro-flow capillary liquid chromatography.








