LC–MS Characterization of Mesquite Flour Constituents
LCGC Asia Pacific
Using a liquid chromatography–mass spectrometry (LC–MS) method in conjunction with two complementary types of chromatographic retention modes-reversed phase and aqueous normal phase-various compounds present in mesquite flour extracts were identified. Because of the diverse types of chemical constituents found in such natural product extracts, a single chromatographic mode may not be sufficient for a comprehensive characterization. However, the combination of reversed-phase and aqueous normal phase LC can encompass a wide range of analyte polarity. This characterization of the composition of mesquite flour could be used in future studies to elucidate the beneficial health effects of its consumption.
Joshua E. Young1, Tina Nguyen2, Charlie Ly2, Sunny Jarman2, Diona Diep2, Cuong Pham2, Joseph J. Pesek2, Maria T. Matyska2, and Gary R. Takeoka3,1MicroSolv Technology Corporation, Leland, North Carolina, USA, 2San José State University, San Jose, California, USA, 3United States Department of Agriculture, Albany, California, USA
Using a liquid chromatography–mass spectrometry (LC–MS) method in conjunction with two complementary types of chromatographic retention modes-reversed phase and aqueous normal phase-various compounds present in mesquite flour extracts were identified. Because of the diverse types of chemical constituents found in such natural product extracts, a single chromatographic mode may not be sufficient for a comprehensive characterization. However, the combination of reversed-phase and aqueous normal phase LC can encompass a wide range of analyte polarity. This characterization of the composition of mesquite flour could be used in future studies to elucidate the beneficial health effects of its consumption.
Mesquite flour is made from the seed pods of shrubs belonging to the Prosopis genus, indigenous to various arid and semiarid ecosystems throughout the Americas, Africa, and Asia. In comparison to wheat-based flour, its consumption has numerous health advantages. Wheat contains a mixture of proteins known as gluten, which imparts chewy, elastic properties to foodstuffs derived from wheat (for example, bread, muffins, cakes, and so forth) However, gluten has little nutritional value and can even be detrimental to certain individuals, such as those with coeliac disease. In this case, ingesting even trace amounts of gluten can induce a serious systemic autoimmune response, necessitating strict adherence to a lifelong gluten-free diet (1). As a legumeâderived food, however, mesquite flour is naturally gluten-free. Hence, it offers a viable dietary alternative for gluten-intolerant individuals who still wish to eat flour-based foodstuffs. For diabetics, its low glycemic index makes it a good source of carbohydrates that can help keep blood sugar levels stable (2). In general, it is high in nutritious proteins as well as in dietary fibre (3,4), the latter of which may be difficult to obtain in some gluten-free diets. Another advantage pertains to the climate of a given area; in certain desert regions where cultivation of wheat or maize crops is difficult, mesquite is often considered to be a staple food.
Consumption of mesquite-derived foods has been linked to antibacterial (5), cardioprotective (6), and anti-inflammatory (7) health effects. Various small molecules that are known to be present in mesquite are thought to play a role in some or all of these properties, including flavonol glycosides, glycosyl flavones, anthocyanins, and ellagic acid derivatives. Hence, to better study these health effects, there is a need for reliable analytical methods for the characterization of compounds found in mesquite flour-based foodstuffs.
This is a nascent topic of food research today and several recent studies have begun to report such characterizations. For example, in their liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis of Chilean Prosopis mesocarp flour, SchmedaâHirschmann and colleagues identified eight anthocyanins and 13 different phenolic compounds (8). Quantitative analysis showed that their antioxidant activity was determined to be 0.82–2.57 g gallic acid equivalents/100 g fresh flour weight. In another recent work, Pérez and colleagues used both LC–MS/MS and high performance liquid chromatography–ultraviolet spectroscopy (HPLC–UV) based methodologies for determining the content of phenolics, flavonoids, anthocyanins, and alkaloids in Prosopis alba and Prosopis nigra extracts (9).
In terms of a total characterization of the constituents of mesquite flour, the primary challenge facing chromatographers is how to retain compounds encompassing the whole range of polarity that may be encountered in a given sample extract. While conventional reversed-phase chromatography may be well-suited to retention of some of the more hydrophobic analytes such as anthocyanins or polyphenols, others that have hydrophilic glycosidic moieties may be more problematic. In this case, aqueous normal phase chromatography may be able to offer a viable solution for adequate retention.
In recent years, aqueous normal phase chromatography has been shown to be a valuable tool in the characterization of food samples. Suited to retention of polar compounds, it can be thought of as a complementary or orthogonal technique to reversedâphase chromatography (10). Hence, both polar and nonpolar compounds can be retained in a complex sample, leading to a more complete characterization of its components. Recent examples of aqueous normal phase chromatography used to retain polar compounds in foodstuffs include histamine in cheese and tuna (11) and sugars in beverages (12).
Along these lines, we performed a qualitative investigation of the chemical constituents of mesquite flour samples using a LC–MS method in conjunction with both reversed-phase and aqueous normal phase retention modes.
Materials and Methods
Materials: Prosopis alba (white mesquite) flour samples were obtained from Argentina. Formic acid was from SigmaâAldrich. Acetonitrile, methanol, and deionized water (HPLC grades) were obtained from GFS Chemicals, Inc.
Instrumentation:
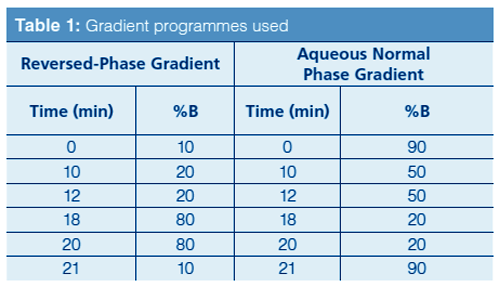
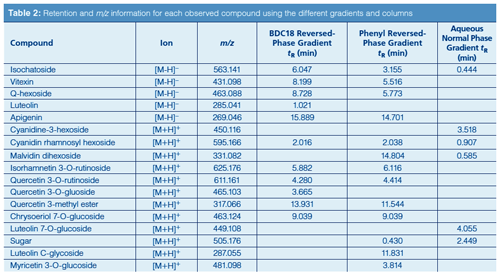
LC–MS: An Agilent 1100 Series LC system, including degasser, binary pump, temperature-controlled autosampler, and temperature-controlled column compartment, was used. The mass spectrometer system was an Agilent Model 6210 MSD time-of-flight (TOF) system with a dual sprayer electrospray ionization (ESI) source. The analytical columns were packed with either a 50 mm × 2.1 mm Phenyl Hydride or a 100 mm × 2.1 mm Bidentate C18 (BDC18) stationary phase, which had a particle diameter of 4 µm and a pore size of 100 Å in either case (MicroSolv Tech. Corp.). The final mobile phase obtained after method development used a gradient: Solvent A was deionized water–0.1% formic acid (v/v) and solvent B was methanol–0.1% formic acid (v/v) for reversedâphase mode or acetonitrile–0.1% formic acid for aqueous normal phase mode. The gradient programmes are shown in Table 1. The flow rate was 0.4 mL/min and the injection volume was 1.0 µL. In the positive and negative ionization modes, the extracted ion chromatogram was obtained for each analyte as the [M + H]+ or [M – H]– ion (see Table 2).
Sample Preparation:
Mesquite Flour Extracts: First, 1.00 g of mesquite flour was weighed on a Mettler Toledo ME204E toploader balance and transferred to a 250-mL beaker. In a graduated cylinder, 15.0 mL of deionized water was measured and transferred to the beaker as well. The mixture was then stirred with a magnetic stir bar on a stir plate for 15 min. Subsequently, it was transferred to a centrifuge tube and centrifuged for 5 min with an International Clinical Centrifuge Model CL centrifuge. The supernatant was collected with a pipet and transferred to a new container. The whole procedure was repeated two more times to obtain a total of 45.0 mL of water-based sample extracts.
The remaining flour in the centrifuge tube was used for additional extractions with 70:30 methanol–deionized water. In a 250 mL beaker, 1.00 g of the wet flour and 15.0 mL 70:30 methanol–deionized water were added. The mixture was then stirred with a magnetic stir bar on a stir plate for 15 min. Subsequently, it was transferred to a centrifuge tube and centrifuged for 5 min. The supernatant was collected with a pipet and transferred to a new container. The whole procedure was repeated two more times to obtain a total of 45.0 mL of methanol–water-based sample extracts.
Both 45.0-mL extracts were then vacuum filtered through a 0.45-μm nylon membrane filter (MicroSolv Tech. Corp.) and transferred to round-bottom flasks. The filtrates were then evaporated using a rubber tube connected to the vacuum line. The filtrates were kept at room temperature until all of the solvent (water or methanol–water) was removed. Aliquots of these filtrates were then used for the LC–MS injections.
Results and Discussion
A summary of the retention times observed for the different analyte ions using both reversed-phase and aqueous normal phase gradients and both column types is shown in Table 2. The table values show that the reversed-phase mode was found to be suitable for most of the analytes that were observed in the mesquite flour extracts. In addition to the specificity afforded by the extracted ion chromatograms (EICs) themselves, chromatographic selectivity between the different peaks spanned a wide distribution of retention times across the chromatograms. With most of the studied compounds, the bidentate C18 column produced slightly greater retention than the phenyl column because of its greater hydrophobicity. However, quercetin 3-O-rutinoside was actually retained slightly longer on the phenyl column. Since the primary difference between the two stationary phases is the ability of the phenyl to participate in π–π interactions, this difference in retention among the two columns seems to suggest that such interactions played a more prominent chromatographic role in the case of quercetin 3-O-rutinoside’s π system.
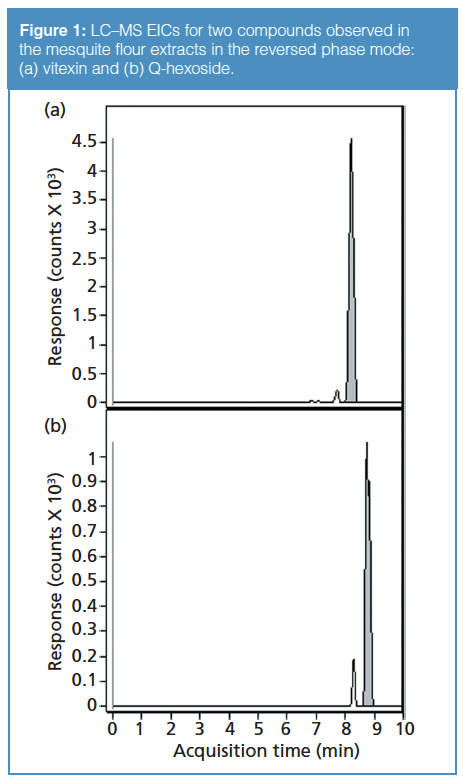
Peak shapes were generally very sharp for all the studied analytes, and no significant tailing was observed. Figure 1 shows representative EICs for two analytes from the mesquite flour extracts in reversed-phase mode using the bidentate column, illustrating the high efficiency of the peaks. In addition to the chromatographic separation itself, the use of mass spectrometry allowed for excellent specificity in isolating individual peaks via the EICs.
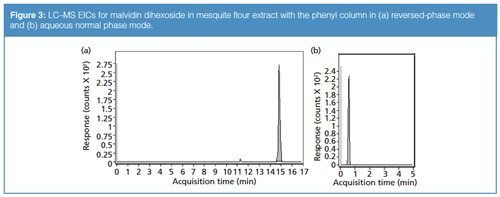
However, some analytes could not be adequately retained using either column in reversed phase, such as the m/z 505.176 sugar compound. In these cases, aqueous normal phase approaches were investigated and often found to be able to retain such compounds. For instance, the same sugar compound that was eluted at the solvent front in reversed-phase mode was found to be eluted at 2.449 min under aqueous normal phase conditions (refer again to Table 2). Cyanidine-3-hexoside and luteolin 7-O-glucoside were also observed to be retained strongly in the aqueous normal phase gradient (see Figure 2). Conversely, malvidin dihexoside was well-retained in reversed-phase mode using the phenyl column but was eluted at the solvent front in the aqueous normal phase mode (Figure 3). This behaviour highlights the complementary nature of the two retention modes: If a compound cannot be retained by one mode, the other may be a viable option for its retention instead. In the comprehensive analysis of complex samples such as mesquite flour extracts-in which many kinds of compounds with disparate structural characteristics may be present- the use of a single chromatographic mode may not be sufficient.
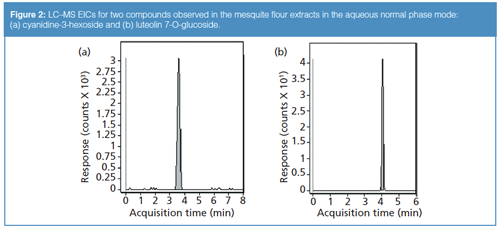
The basis for this orthogonality of retention pertains to the differences in the reversed-phase and aqueous normal phase retention mechanisms. In reversed-phase mode, the separation is driven by hydrophobic solute–stationary phase interactions, with the most polar compounds eluted first. In aqueous normal phase, however, the inverse is true: The most polar compounds are generally retained the strongest. Hence, the two chromatographic modes together can often be suitable for the retention of a wide range of compounds found in a sample, ranging from the highly hydrophilic to the hydrophobic.
For all of the investigated aqueous normal phase separations, the phenyl column was chosen over the bidentate C18. The rationale for this decision was that the bidentate C18 ligand is thought to be too hydrophobic for effective aqueous normal phase retention in many applications. Steric hindrance from the bulky C18 ligand could also obstruct solute adsorption on the silica hydride surface, thereby inhibiting aqueous normal phase retention. In either case, low retention in the aqueous normal phase mode was expected for a bidentate C18 phase, so the phenyl was used exclusively in these aspects of the study.
Identification of a wide variety of mesquite flour components was made possible by the use of these two orthogonal retention modes. Future studies can make use of this information in an effort to more fully elucidate the mechanisms by which mesquite consumption can lead to antioxidant, cardioprotective, analgesic, and antibacterial therapeutic effects.
Conclusion
The compounds identified in the P. alba sample of mesquite flour were different than what was reported in previous publications. This may be because of the combination of both reversed-phase and aqueous normal phase modes to achieve a more complete characterization of the sample constituents than what was possible with one retention mode alone. Both columns used in the analysis were suitable for applications in food analysis of mesquite flour. The phenyl phase selectivity was mostly comparable to that of the bidentate C18, with differences mainly attributable to greater hydrophobicity of the bidentate C18 and π–π interactions of the phenyl. In the case of the phenyl phase, dual retention capabilities (reversed phase and aqueous normal phase) were shown.
Acknowledgements
The authors would like to acknowledge Microsolv Technology Corporation for donation of the analytical columns used in this study and the U.S. Department of Agriculture (USDA) for the donation of the standards and flour.
References
- G. Serena, S. Camhi, C. Sturgeon, S. Yan, and A. Fasano, Nutrients7, 7143–7162 (2015).
- N. Sharma, V. Garg, and A. Paul, Indian J. Clin. Biochem.25, 193–200 (2010).
- D. Meyer, R. Becker, M.R. Gummann, P. Vohra, H. Neukom, and R.M. Saunders, J. Agric. Food Chem. 34, 914–919 (1986).
- P. Felker, G. Takeoka, and L. Dao, Food Rev. Int. 29, 49–66 (2013).
- S. Tajbakhsh, A. Barmak, F. Vakhshiteh, and M. Gharibi, J. Clin. Diagn. Res.9, DC13–DC15 (2015).
- B. Huisamen, C. George, D. Dietrich, and S. Genade, Cardiovasc. J. Afr. 24, 10–16 (2013).
- M.A. Abodola, M.F. Lutfi, A.O. Bakhiet, and A.H. Mohamed, Int. J. Health Sci.9, 266–271 (2015).
- G. Schmeda-Hirschmann, C. Quispe, M.C. Soriano, C. Theoduloz, F. Jiménez-Aspée, M.J. Pérez, A.S. Cuello, and M.I. Isla, Molecules20, 7017–7033 (2015).
- M.J. Pérez, A.S. Cuello, I.C. Zampini, R.M. Ordoñez, M.R. Alberto, C. Quispe, G. Schmeda-Hirschmann, and M.I. Isla, Food Res. Int. 64, 762–771 (2014).
- J.J. Pesek and M.T. Matyska, J. Sep. Sci. 32, 3999–4011 (2009).
- A. Dang, J.J. Pesek, and M.T. Matyska, Food Chem.141, 4226–4230 (2013).
- J.E. Young, J.J. Pesek, and M.T. Matyska, Curr. Nutr. Food Sci.12, 125–131 (2016).
Joshua E. Young is with MicroSolv Technology Corporation in Leland, North Carolina, USA. Tina Nguyen, Charlie Ly, Sunny Jarman, Diona Diep, Cuong Pham, Joseph J. Pesek, and Maria T. Matyska are with San José State University in San Jose, California, USA. Gary R. Takeoka is with the United States Department of Agriculture in Albany, California, USA.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








