Ionization Revisited
LCGC Europe
Electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are now among the most commonly used techniques for creating ions, especially from small-molecule compounds in solution. They have become so familiar that now many articles only refer to them briefly. Yet each technique has dramatic predictive strength on the outcome and limits of an analysis.
Electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are now among the most commonly used techniques for creating ions, especially from small-molecule compounds in solution. They have become so familiar that now many articles only refer to them briefly. Yet each technique has dramatic predictive strength on the outcome and limits of an analysis.
As a related aside to this topic, you might read an earlier column,1 which describes the non-liquid chromatography (LC) ionization methods publicised in recent years: desorptive electrospray (DESI) and direct analysis real time (DART). Also, from www.CoSMoScience.org, you can download a PDF version of Charles McEwen's (DuPont, Wilmington, Delaware, USA) excellent presentation, which he delivered at last year's Conference on Small Molecule Science at San Diego, California, USA. That presentation focuses on gas chromatography (GC) ionization that was developed without significantly modifying existing ESI sources. Of special interest is McEwen's comparison between APCI as the ionization mechanism and APPI. Briefly, a reduced vapour load and lamp energy combine to make both a sensitive and specific ionization technique — as sensitive as APCI but with less noise. The work also serves as a cautionary tale depicting how ions created from the same analytes can look quite different depending upon the ionization technique you use.
Practical and theoretical knowledge culled over the last decade of developing and applying atmospheric ionization techniques teaches us some lessons. Liquid, while a necessary intermediary in separating and transporting analytes and creating ions, is also a vestigial remnant of the condensed-phase LC process. To paraphrase from McEwen's presentation,2 solvent in the gas phase limits ionization to molecules that are more basic than the solvent (except in APPI, which is not acid–base but nonetheless solvent-mediated). Therefore, removing solvent and water vapour from the ionization region increases the number of compounds ionizable by the most convenient methods performed at atmosphere.
Atmospheric Pressure Ionization: Electrospray
Work performed in the early 1990s has only recently yielded a more unified understanding of the mechanisms involved in ion production. The transfer mechanism of potential from liquid to analyte, which creates ions, had been a topic of controversy. Richard Cole's article in 2000 is still one of the better examinations of the two most popular theories.3 The charge residue mechanism, first proposed by Dole in 1968,4 theorizes a chain of events where, as droplets evaporate, the surface tension on each cannot ultimately oppose the repulsive forces from the imposed charge and each droplet explodes into many smaller droplets. These coulombic fissions occur until droplets containing a single analyte ion remain. A gas-phase ion forms when solvent from the last droplet evaporates.
Iribarne and Thomson proposed the ion evaporation mechanism in 1976 as an alternative model.5 Also in this theory, small droplets are formed by coulombic fission. However, the electric field strength at the surface of the droplet is thought to be high enough to make it energetically favourable for solvated ions to leave the droplet surface and transfer directly into the gas phase. It is possible, as Cole argues, that the two mechanisms actually might work in concert. The charge residue mechanism dominates for masses higher than 3000 Da; the ion evaporation dominates for lower masses.
As practitioners, we have come to accept that ESI works. But understanding how ions are liberated from our liquid mobile phase in the gas-phase transition will help us understand and diagnose issues such as lack of expected sensitivity and ion suppression. The ESI probe, or device, is typically a conductive capillary, usually made of stainless steel, through which the solvated analyte flows. The capillary, contained within a larger-bore cylinder, allows a concentric nitrogen flow applied to the aerosol at its exit point. The added shear forces of the gas and heat transmitted from adjacent supplemental devices, or direct heating of the gas itself, enhances formation of aerosol droplets. The solvent leaves the ESI probe carrying a net ionic charge.
The liquid, usually introduced via an LC process, enters the ESI probe in a state of charge balance. For ESI to be continuous, the solution must be charged by electrochemical reactions. In those reactions, ions are transferred from a conductive surface, which leads to pH changes among other effects. The theory proposes that positive droplets leave the spray and electrons are accepted by the electrode (oxidation); the reverse occurs in negative mode. The surface area of the electroactive electrode, the magnitude of the current and the nature of the chemical species and their electrode potentials all exert an effect.
Overall, ESI is an efficient process. However, the activation energy varies as does the combined energy differences for individual species. In general, although the ion-creation process is considered highly efficient, transferring the ions generated at atmosphere into the vacuum system and ultimately detecting them, is not. To detect a single ion can take as many as 5000 molecules in the original condensed-phase solution. The efficiency depends greatly upon the flow-rate of the original solution as well as the chemical characteristics of the molecules themselves.
The solution's flow-rate and the current applied to the solution define limits for each droplet. Competition between molecules occurs as does suppression of analytes of interest. When considering the prospects for combining ionization mechanisms in the same-source environment, chemical emendations that prove effective for one compound might prove inimical to others in the same mixture. The elements in common are the ability to initiate a liquid droplet aerosol that, in the presence of heat, promotes desolvation of the droplets. You can find an excellent review of our current understanding of these mechanisms in a recent article by Kebarle.6
Atmospheric pressure ionization — chemical ionization: Although work demonstrating APCI was published in parallel with ESI it was not widely adopted until ESI was commercialized following Fenn's work in 1985.7 Horning first introduced APCI in 1973 to analyse volatile compounds,8 using various introduction techniques, one of which was LC.9 The adjunctive capability of APCI permits analytes that resist converting to gas-phase ions by ESI — that is, the less polar and more volatile ones — to be introduced to a mass spectrometer from a condensed phase or liquid stream.
Unlike ESI, APCI transfers neutral analytes into the gas phase by vapourizing the introduced liquid in a heated gas stream. Chemical ionization relies on the transfer of charged species between a reagent ion and a target molecule to produce a target ion that can be mass analysed. Most commonly in positive ion mode, an adduct forms between the target molecule and the small H+ ion although adducts with salts are common as well. As an example, ammonium (M+NH4+ ) when the weak acid–weak base salt ammonium acetate is present in the mobile phase. At higher salt concentrations, competition between the protonated form and ammoniated form can produce a decreased response for both. The maximum number of ions capable of formation by APCI is much higher than in ESI, because reagent ions are formed redundantly.10
The liquid is pushed through a nonconductive tube, usually of fused-silica glass, around which a nebulizing gas flows. The resultant fine droplets collide with the inner, heated wall of a tube or probe that extends beyond the end of the non-conductive tube, converting to the gas phase. This form of ionization is often performed at much greater linear velocities than those provided by the high performance liquid chromatography (HPLC) flow-rates associated with electrospray (closer to 1 mL/min versus electrospray, where decreasing the flow can improve performance).
The desolvated analyte molecules are then ionized via chemical ionization. The ionizing potential is applied, not through the liquid as in ESI, but at the tip of a needle as a plasma or corona through which the droplets pass. In effect, the mobile phase acts as an intermediary transferring the charge to the analyte. Hence, the name given APCI early on: "solvent-mediated electrospray".
Nucleate boiling is more typical of contemporary designs where a heated chamber extends as much as a few inches beyond the aerosol outlet encompassing the spray. Latent heat measured by a thermocouple placed at an arbitrary point in the device can measure as much as 600 °C as part of the temperature control feedback loop, although the analyte encased in the aerosol droplet experiences little of this heat directly. Gas temperature measured at the outlet of such a device might only be 20% of the heat measured at input. Unlike some gases commonly used in mass spectrometry (MS), such as helium, nitrogen's heat induction capacity is more instrumental in providing shear forces for desolvating the droplets. The heavier nitrogen is pumped away more efficiently, allowing more latitude in vacuum design and it is an easily generated, less expensive commodity.
The droplet's time on the heated surface (residence time) before its nucleation and the time the droplet takes to travel the length of the heated chamber is relatively brief. Therefore, the "protective" liquid layer usually preserves the analyte from pyrolytic degradation. In some instances, for example, N-oxide structures and some metabolites, like glucuronides and sulphates, an inherent and increased sensitivity to thermal degradation yields the original drug structure instead.11,12 Thermal degradation in itself can prove a useful analytical tool when well characterized.13
Atmospheric pressure ionization — photo-ionization: APPI is a relatively recent commercial development in analytical mass spectrometry. So far, spectrometrists have used it to complement APCI and ESI operation and to extend the range of molecules efficiently ionized in LC–MS analyses.14,15 Jack Syage, CEO and founder of Syagen Technology (Tustin, California, USA), explains the ion most frequently observed by APPI is typically protonated. As such, it is similar to ions produced via ESI. The protonation indicates that a chemical reaction follows the photoionization event. This is true regardless of whether the protonation occurs in the presence of reagent dopant ions or as a product of direct APPI.16 Cases recently published such as recombinant ions being formed in the presence of acetonitrile indicate there is more to learn. At 12.19 eV (similar to 12.29 eV for water) acetonitrile is well above the ionization energy of the krypton lamp used in APPI (10 eV) where most analytes of interest are well below that yet anomalies have been noted.17 In some instances, which seem to correlate with molecules of low proton affinity, M+ is observed much like the result of high-energy processes such as electron ionization (EI). However, we do not know whether this process dominates at atmospheric pressure. There are two general mechanisms possible to explain the product of MH+ from M:
- M + R+ Φ MH+ + R (–H): protonation by R+ , a charge carrier;
- M+ + S Φ MH+ + S(–H): hydrogen atom abstraction from S, a protic molecule.
This protonation mechanism is the basis for both APCI and ESI. Much of the work published on APPI assumes a dual-source approach, where the existing API source — usually an APCI design — generates the aerosol. A krypton gas-filled lamp is positioned to transmit 10 eV photons at the cross-section of the aerosol, in line with the ion inlet. As in APCI, the desolvation required for APPI must exceed that required for ESI because vapour can interfere in an analogous way with respect to the high flow-rate artifacts found in other forms of ionization. Photoionization has been used widely in gas chromatography applications since the 1970s. Hence, the application pursued by McEwen as demonstrated at CoSMoS and mentioned previously. Enhanced source desolvation developments and the inability to ionize a significant number of compounds via ESI gave HPLC–APPI-MS a commercial impetus. At about the turn of the new century, those developments included an increased need to analyse compounds not easily amenable to ESI as well as compounds typically out of APCI range, like steroids and polycyclic aromatic hydrocarbons (PAHs).
The Trouble with Liquid
Liquid's physicochemical properties are the dominant influence on ESI behaviours. Reducing liquid interference yields improved response linearity in mass spectrometer interfaces.18 Through whatever mechanism ion formation proceeds, if we accept the electrohydrodynamic theory that ions are produced when droplets are reduced to approximately a 10 µm radius, the prospect for a larger population of ions produced at any given point improves when a more homogenous population of smaller-diameter droplets is initially produced. The surface properties of the liquid and the volume admitted to the mass spectrometer source enclosure determine the number of ions liberated from the liquid per unit time. Reduced surface tension and reduced flow-rate are primary factors. Reducing the flow-rate to the nanolitre-per-minute range also has been postulated as a means to reducing signal suppression, possibly by generating smaller, more highly charged droplets that better tolerate nonvolatile salts.19 Even early work with particle beam technology demonstrated how reducing the vapour load and producing an improved cross section of smaller droplets improved linearity and sensitivity by removing the impediment of unnecessary vapour (Figure 1).20
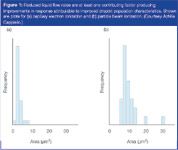
Figure 1
A mass spectrometer's ion-source geometry and properties related to heat propagation and gas dynamics are less easily compared between competing designs. In practice, a design suits a number of practical (often market-driven) requirements, including upper and lower liquid flow-rates, tolerance to non-volatile components in the liquid and aerosol production and maintenance. Once the API aerosol is initiated, further desolvation must occur within the geometry of the ion source. Dispersion of droplets away from the ion inlet and incomplete droplet desolvation play a major role in ion losses. Both are controlled by fundamental gas dynamic principles, which can be studied via computational fluid dynamic models. Both are modelled analytically, which ultimately teaches us that the physical processes of gas entrainment and recirculation dominates the trajectory of the aerosols. Various equations have been derived to characterize source behaviours, for example Busman for APCI space charge limitations in a spherical geometry.21
A commercially viable form of ionization used in LC–MS in the early 1990s was referred to as thermospray.22 The technique, which has since been supplanted by ESI, produced ions through desolvation of the liquid stream using the shear forces associated with largely aqueous mixtures at high flow-rates (achieving high linear velocities). Heating the conducting tube or probe to just below the point where total vaporization occurred at the outlet allowed final desolvation to occur in the reduced pressure region, with the resulting ions produced from the droplets in position to be sampled with good efficiency. The optimal flow-rate of 1 mL/min into a vacuum system required very high capacity pumping and trapping systems to remove the resulting vapour. Because this technique worked with a highly aqueous mobile phase an approximate illustration taken at atmospheric equivalent with water indicates that a 1 mL/min flow produces 1 L/min of vapour.
ESI works largely with compounds that exhibit little or no thermally achievable vapour pressure. APCI and APPI extend the utility of API instruments to enhance sensitivity for less polar and more volatile compounds where ESI is not as effective. Some compounds can produce ions without undergoing an additional form of ionization. "Mixed mode" behaviours have been noted where removing the means of external ionization, such as an APCI needle, resulted in thermally induced ions.23 Such ions are akin to those produced by thermospray. However, they are produced at atmosphere rather than under reduced pressure (the case with thermospray) and pneumatically assisted ESI.
A Few Observations
Adjusting solvent characteristics — that is, increasing or decreasing pH to shift protonation tendencies or change a liquid's surfactant properties to promote the liberation of ions from droplet surfaces — often promotes electrospray ions from an analyte previously thought intractable in terms of the ESI mechanism of ion production.
Here are some other lessons from our studies:
- Increasing the desolvation heat while increasing the carrier gas flow can bring about diminishing returns. The increase in pressure, the change in gas turbulence conducting heat away from the chamber and other factors can increase noise and reduce the likelihood that an ion of interest will appear to increase in analysis. Thus, the background ion population might not act colinearly with the ion of interest. Attempts to optimize the appearance of that ion can result in a more intense peak but reduced signal-to-noise ratio.
- Turning off the current to your APCI needle or energy to your APPI lamp might demonstrate thermal ions are being produced and in fact you might find the APCI plasma is actually suppressing them.
- Just as no single analytical instrument works for all studies, no single ion source satisfies all conditions. A source might operate well at extremely high flow-rates, like those suited to monolithic columns, but operate far less effectively at low flow-rates.
Follow-up From Readers
The recent column on sources of information from the internet (October 2006) spurred some useful comments such as this one from the UK: "I've been reading your columns in LCGC Europe with interest and saw your article on stms this month. I'm not sure if you're aware but there's also the option to access this usenet group via Google at http://groups.google.com/group/sci.techniques.mass-spec. There you've also got all the power of Google searches at your fingertips. To post, you have to sign up to Google, but it's free and there's other good stuff from Google if you have an account. You can also search for MS-related posts in other usenet groups easily through Google."
"MS in Practice" editor Michael P. Balogh is principal scientist LC–MS technology development at Waters Corporation (Milford, Massachusetts, USA); an adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island, USA); and a member of LCGC Europe's Editorial Advisory Board. Direct correspondence about this column to "MS in Practice", LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK or e-mail dhills@advanstar.com
References
1. M.P. Balogh, LCGC Eur., 19(7), 412–417 (2006).
2. C.N. McEwen, High Sensitivity Analysis of Volatile and Semi-volatile Compounds using ASAP and GC–MS on an LC–MS Instrument, http://www.cosmos2006.org/Presentations_2006/HighSensitivityAnalysis.pdf
3. R. Cole, J. Am. Soc. Mass Spectrom., 35, 763–772 (2000).
4. M. Dole et al., J. Chrom. Phys., 49, 2240–2249 (1968).
5. J.V. Iribarne and B.A. Thomson, J. Chem. Phys., 64, 2287 (1976).
6. P. Kebarle, J. Am. Soc. Mass Spectrom., 35, 804–817 (2000).
7. C.M. Whitehouse et al., Anal. Chem., 57, 675–679 (1985).
8. E.C. Horning et al., Anal. Chem., 45, 936–943 (1973).
9. D.I. Carroll et al., Anal. Chem., 47, 2369–2373 (1975).
10. C.H. Bruins et al., J. Chrom. A, 863, 115–122 (1999).
11. W. Tong et al., Rapid Commun. Mass Spectrom., 15, 2085 (2001).
12. D.Q. Liu and T. Pereira, Rapid Commun. Mass. Spectrom., 16, 142 (2002).
13. P. Siegel, T. Karancsi, Application of Thermal Decomposition for Structure Elucidation during APCI Experiments, Advances in Mass Spectrometry, 14 (1998), proceedings from the 14th International Mass Spec Conf, Tampere, Finland, August (1997), E.J. Karjalainen et al., Eds.
14. J.A. Syage, M.D. Evans and K.A. Hanold, Amer. Lab., 32, 24–29 (2000).
15. D.B. Robb, T.R. Covey and A.P. Bruins, Anal. Chem., 72, 3653–3659 (2000).
16. J.A. Syage, J. Am. Soc. Mass Spectrom., 15(11), 1521–1533 (2004).
17. E. Marottaa et al., Int. J. Mass Spectrom., 228, 841–849 (2003).
18. A. Cappiello et al., Anal. Chem., 72(16), 3841–3846 (2000).
19. E.T. Gangl et al., Anal. Chem., 73, 5635–5644 (2001).
20. A. Cappiello et al., Mass Spec. Rev., 20(2), 88–104 (2001),
21. M. Busman, J. Sunner and C.R. Vogel, J. Am. Soc. Mass Spectrom., 2(1), 1–10 (1991).
22. C.R. Blakeley and M.L. Vestal, Anal. Chem., 55, 750–754 (1983).
23. P. Brown et al., Thermal Spray is Back: A Modified APCI Interface on the LC–MS–MS Bioanalysis of Famotidine in Human EDTA Plasma, 48th ASMS Conference on Mass Spectrometry and Allied Topics, Long Beach, California, June (2000).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













