Investigating Decomposition Odor in a Tropical Climate by Comprehensive Two-Dimensional Gas Chromatography (GC×GC) Coupled with Mass Spectrometry (MS) and Flame Ionization Detection (FID)
During decomposition of mammalian remains, volatile organic compounds (VOCs) are released into the environment. Comprehensive two-dimensional gas chromatography (GC×GC) assists in analyzing these VOCs with its increased separation power and sensitivity. This study analyzes the temporal changes in decomposition VOCs from human analogs in a tropical climate. For this specific application, the use of a gas chromatograph coupled with a quadrupole mass spectrometer (qMS) and simultaneous flame ionization detector (FID) retrofitted with a reverse fill/flush (RFF) flow modulator is reported for the first time. The method was used to examine postmortem processes to monitor chemical changes from pig carcasses. Approximately 30 compounds were tentatively identified from the decomposing remains, representing consistent trends in the decomposition VOC profile at a new location. As interest increases worldwide in collecting VOC samples as evidence in forensic casework, this type of instrumentation could become a valuable tool in crime laboratories.
The decomposition process of human and animal remains involves major changes and initiates the production of many by-products. Some of these by-products are organic in nature and originate from biochemical reactions that break down macromolecules within biological tissue. Because of enzymatic reactions and microbial processes, the molecules gradually break down, resulting in a large number of volatile organic compounds (VOCs). In forensic science, this collection of VOCs is referred to as decomposition odor, and can be used in forensic tracing for various purposes. For instance, decomposition odors can be detected to support the presence of a body at a particular location (1). It has been hypothesized that their presence can also be correlated to the length of time since death when estimating the post-mortem interval (PMI) (2). This type of information is important in establishing a series of events in the circumstances surrounding a death of a human or animal, which can be critical information in a forensic investigation. Furthermore, the improved understanding of the formation and evolution of decomposition odor is of high interest to handlers of cadaver-detection dogs to improve training practices in search and rescue operations (3).
From a chemical point of view, decomposition odor represents a complex mixture of gaseous molecules and its complete characterization requires a powerful analytical technique. In the early stages of decomposition odor research, one-dimensional gas chromatography coupled to mass spectrometry (1D GC–MS) was most often used (4,5). However, the limited separation power was challenged by the complexity of these samples and complete resolution of the compounds was often not achieved, which can lead to significant challenges in understanding the compositions of decomposition odor, the trends in compound abundance over the duration of the decomposition process, and implications of decomposition odor production given different external variables. Ultimately, using 1D GC–MS is problematic because it hinders the ability of this work to reach critical areas of need that rely on understanding decomposition odor, such as helping to locate missing persons or homicide victims.
In recent years, a general shift from 1D-GC to comprehensive two-dimensional gas chromatography (GC×GC) has been observed in this field (6–8). The general shift to comprehensive two-dimensional gas chromatography (GC×GC) happened because of the superior peak capacity, sensitivity, and selectivity of GC×GC (9). In addition, GC×GC instrumentation allows more effective management of the large dynamic range encountered in decomposition VOC studies (9). This development has been followed with more accurate concentration measurements and higher confidence in decomposition VOC identification. Currently, decomposition odor is largely studied using GC×GC in research laboratories, which may be partly because of the lengthy and challenging process of adapting a novel analytical technique to the needs of a modern forensic science laboratory. In addition, cost and time investment for method validation through laboratory accreditation, as well as acquisition and maintenance costs, might impede its implementation. However, recent technical advances led to commercial development of new GC×GC equipment configurations with some of them having the potential to be a tool in forensic laboratories (8). In this study, a GC×GC instrument with a quadrupole mass spectrometer (qMS) and simultaneous flame ionization detector (FID) with a reverse fill/flush (RFF) flow modulator was employed to analyze VOCs emitted by decomposing human analogs.
In the past, the decomposition VOC profile has been studied on different continents, including locations in Europe, North America, and Australia. However, most studies are performed in humid subtropical or humid continental climates. Table I lists location and climate zones according to the Köppen-Geiger classification (10) for all longitudinal decomposition studies, with more than one rep- licate investigating the VOC profile from human or animal remains. In spite of some local climatic differences, some general trends could be established between these studies (11,12). However, the local environment heavily impacts decomposition outcomes; therefore, it is important to understand decomposition odor evolution in a variety of climate zones. The purpose of this study was to perform the first decomposition odor study in a tropical region using comprehensive two-dimensional gas chromatography-single quadrupole mass spectrometry and a flame ionization detector (GC×GC–qMS/FID). Specifically, the tropical savanna climate experienced in Honolulu, Hawaii, was chosen. Honolulu is a contrasting location to areas that have been previously studied because of its year-round, consistently warm temperatures and its minimal temperature difference throughout the day. The tropical savanna is the second-most common climate type in the world (following hot desert), and these regions experience many climate events, such as tropical storms, and these types of climate events contribute to a need for forensic search and recovery. Therefore, it is very important to improve our understanding of decomposition odor in these regions. This study reports on a GC×GC–qMS/FID-based method that was applied to examine the postmortem decomposition process to monitor chemical changes of surface deposited pig carcasses on a decomposition facility in a tropical climate.
Materials and Methods
Decomposition Trial
This research was conducted in April 2019. The trial was performed using three swine (Sus scrofa domesticus) carcasses, each weighing approximately 30 kg. The pigs were part of the same breeding program; therefore, they were sufficiently similar in terms of body composition to represent biological replicates. The carcasses were purchased from a licensed abattoir postmortem. Therefore, an animal ethics approval was not required because the experimental subjects were not killed specifically for the purposes of research. The carcasses were transported to the field site within an hour of death. They were placed in an outdoor decomposition site located in Palolo Valley, Honolulu, Oahu, Hawaii, previously described by Chun and others (28). This particular site constantly experiences a tropical savanna climate (10). The vegetation at the moderately steep site is representative of a tropical savanna ecosystem on Oahu; it is rocky and dominated by guinea grass (Megathyrsus maximus) with night blooming cereus (Hylocereus undatus), aloe (Aloe spp.), and carrion plants (Stapelia gigantia). Figure 1 shows an aerial view of the outdoor decomposition site. Three control sites were designated, containing the same soil and vegetation but no decomposing remains.
FIGURE 1: Aerial view of the outdoor decomposition site on Oahu, Hawaii.

VOC Sampling of Pig Carcasses
A stainless steel hood (dimensions, 122 × 91 × 56 cm; volume, 622 L) was placed over the pig carcasses. After an equilibration time of 15 min, VOCs in the air above each of the three pig carcasses were collected by active sampling. For this purpose, a constant flow air pump was attached to the sampling port of the hood. VOCs were collected onto sorbent tubes containing Tenax TA and Carbograph 5TD. A flow rate of 100 mL/min was used for 10 min, pumping a total volume of 1 L of headspace gases through the sorbent tubes. Starting from the day of the deposition (Day 0, notated as D0), samples were taken every second day for two weeks (D0, D2, D4, D6, D8, D10, D12, and D14).
The same experimental design was used to collect samples from the headspace above three control sites on each sampling day. Control sites were located in a similar region to the experimental samples, with representative soil and vegetation, but did not contain decomposing remains. Control sites were located upslope of the carcass samples to prevent any contamination from groundwater, and were also located away from areas where carrion plants occasionally flowered. Carrion plants did not flower during the course of this experiment, but sample collection occurred in an isolated manner with the stainless steel hood to prevent this source of potential impact. All sample collection tubes were capped with long-term storage caps and stored in an air-tight, sealed glass jar for transport to and from the field site. Field blank samples were collected on each sampling day by opening a tube at the site for 10 s and recapping. The field blank was stored alongside the samples and represented any compounds that would be present from the sampling protocol that are not attributed to the site itself. Further details of this sampling procedure and the necessity for implementing these quality assurance measures are detailed in the literature (19).
All sample tubes were injected with a deuterated internal standard (1 μL of 100 ng/μL chlorobenzene-d5, Sigma Aldrich) in high performance liquid chromatography (HPLC)-grade methanol (J.T. Baker). A C7-C30 saturated alkane standard (Sigma Aldrich) was injected using 1 μL of 100 ng/μL of hexane (Sigma-Aldrich) prior to the first sample injection and immediately following the last sample injection to enable the calculation of linear retention indices.
GC×GC–qMS/FID Analysis
Sample analysis was carried out using a Unity-xr thermal desorber (Markes International Ltd) and a Thermo Scientific Trace 1300 gas chromatograph and flame ionization detector with an ISQ 7000 single quadrupole with an insight flow modulator (SepSolve Analytical Ltd). The qMS data were used to tentatively identify compounds comparing the mass spectra to the National Institute of Standards and Technology (NIST) library and the FID data for quantification (8,29).
Each sample underwent a two-step desorption: Primary desorption of the sample took place with a trap flow of 50 mL/min and split flow of 20 mL/min at 300 °C for 5 min following a 1-min nitrogen dry purge. The sample was re-condensed at −10 °C on a general purpose carbon cold trap (Markes International Ltd). The cold trap was then rapidly heated for secondary desorption at 320 °C for 3 min following another 1-min nitrogen dry purge.
An Rxi-624Sil MS column (30 m × 0.25 mm i.d. × 1.4 μm film thickness) and a Stabilwax (5 m × 0.25 mm i.d. × 0.25 μm film thickness) were used in the first and second dimension, respectively (Restek Corporation).
The flow rate in the first dimension column was 1 mL/min, the auxiliary gas flow was 20.14 mL/min, and the flow rate in the bleed line (5 m × 0.1 mm i.d.) was 1.03 mL/min. The loop dimensions were 0.53 mm i.d. × 1133 mm, resulting in a loop volume of 25 μL. The modulation period was 2.5 s and the flush time was 100 ms. The flow rate in the second dimension column was 17.9 mL/min. The flow was split with a ratio of 4.5:1 between the FID and mass spectrometer.
The GC oven started at an initial temperature of 65 °C and was increased to a final temperature of 250 °C at a rate of 3 °C/min and held for 5 min. The transfer line and the ion source temperature were held at 280 °C. The qMS was operated in full electron ionization (EI) scan mode with a mass range of 40–300 m/z operating with a rate of ~41.5 scan/s. The FID was operated with 350 mL/min ultra-zero grade air (Airgas), 4 mL/min ultra high purity nitrogen as makeup gas (Airgas), and 35 mL/min ultra high purity hydrogen (Airgas). The temperature of the FID was set at 250 °C. The acquisition rate of the FID was 120 Hz. Instrument control was performed using Chromeleon 7 version 7.2.9 (Thermo Scientific).
Data Processing
Data acquisition was performed for both data sets using the Thermo Scientific Chromeleon CDS software. GC–qMS data were processed with the same software. GC×GC–qMS .raw files were exported, converted to the .cdf format, and imported into the ChromSpace software (SepSolve Analytical Ltd) for processing. GC×GC-FID files were exported as .cdf files and imported into the ChromSpace software (SepSolve Analytical Ltd) for processing.
GC×GC–qMS
Dynamic baseline correction was performed on imported .cdf files with a peak width of 0.4 s. Stencils for the peaks of interest were obtained by applying the curve-fitting algorithm for peak integration with a 3-point Gaussian smoothing function. Stencils are defined regions on the contour plot where peaks are searched for, similar to retention windows in 1D-GC data. The minimum peak area was 5000, the minimum peak height was 50000, and the minimum peak width was 0.001. Parameters for peak merging included a tolerance of 2%, an overlap of 2%, an intensity of 2%, and a correlation of 0.5. Subpeak apex windows for fronting and tailing were set to 2% for both low and high peak maxima. Peak response for qMS data is recorded in counts.
GC×GC-FID
Top-hat baseline correction was used on imported .cdf files using a peak width of 0.4 s. Stencils obtained from GC×GC–qMS data processing method were transferred to FID files and the stencil was transformed manually to align over FID peaks. Peak detection was performed using the local regions of interest produced by these stencils with a minimum peak area of 1, a minimum peak height of 0, and a minimum peak width of 0. FID peak areas, normalized using the area of the internal standard, were used to examine temporal trends between samples. Peak response for FID data is recorded in pA.
Results and Discussion
Chemical Profile
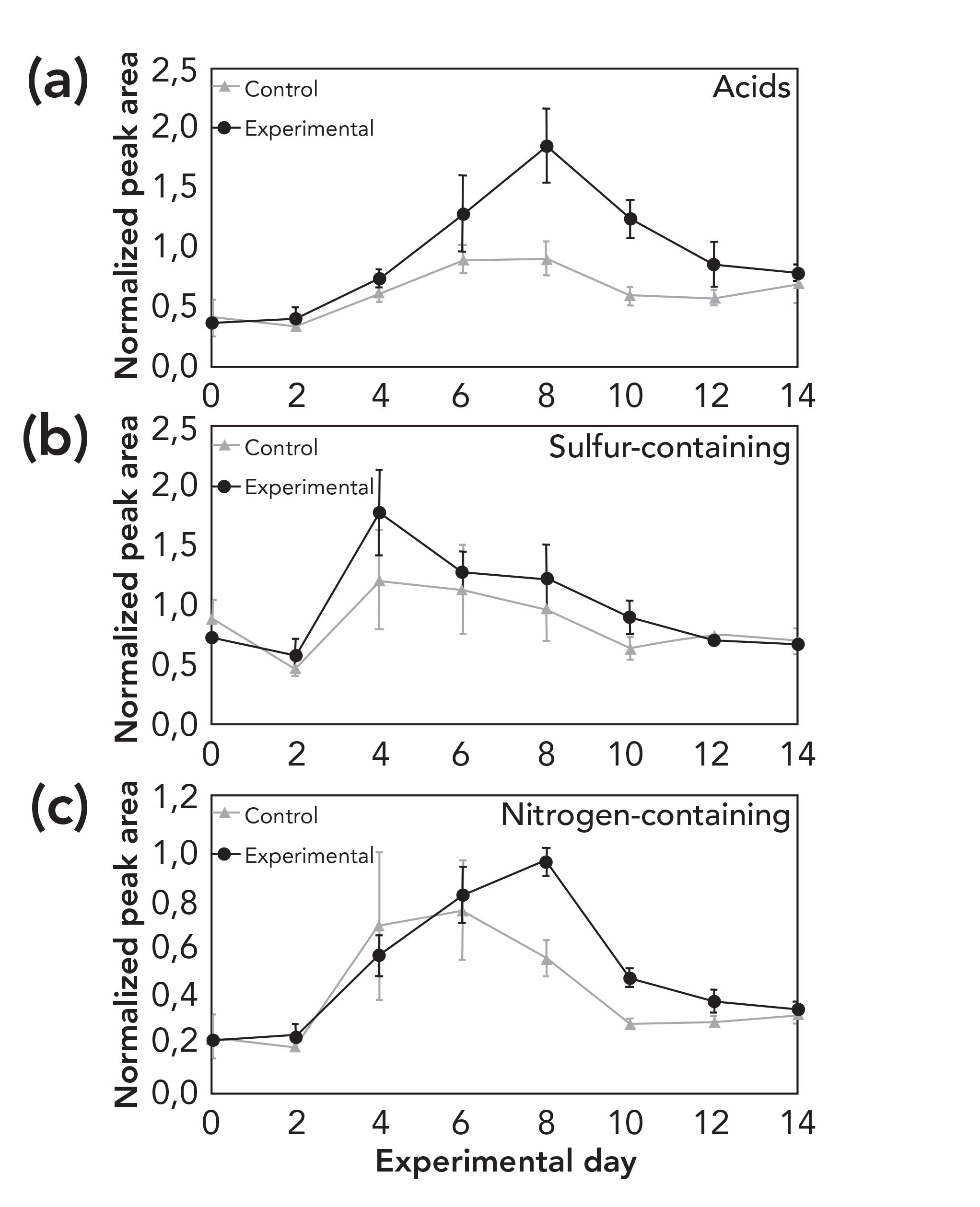
Figure 2 displays two chromatograms as examples of a typical VOC profile of the fresh stage (A) and active decay stage (B) of decomposition. For each sampling day, the VOC profile was characterized and compounds were assigned to one of the major chemical classes (acids, sulfur-containing compounds, ester, nitrogen-containing compounds, heterocyclic, alcohol, ketone, aldehyde, and other). Figure 2 demonstrates the complexity of the initial fresh profile, which largely contributes to the environmental organic matter in the soil and vegetation surrounding the remains, with some contributions made by the skin microbiota. Figure 3 displays the temporal changes between day 0 and day 14 of the trial. Figure 4 provides a more detailed view of acids, and of sulfur- and nitrogen-containing compounds. The chemical classes reported in this study have been previously reported as part of the decomposition profile (30). However, organic matter is present in all soil and therefore various compound classes show elevated levels also at control sites. Because of the fact that alkanes and aromatics were also present at high levels in the control samples and followed a similar trend, they were not included in these figures. Temporal trends appeared to exist for acids, sulfur-containing compounds, ester, and nitrogen-containing compounds. In Canada, Stadler and others analyzed VOCs from surface-deposited pig carcasses and found an increase of sulfur-containing compounds during the early stage of decomposition (26,27). In Greece, Agapiou and others reported a peak of sulfur-containing compounds on the first day of their trial with buried pig carcasses (24). Forbes and Perrault in Australia also monitored a higher presence of various sulfur-compounds; however, no values for their relative abundance are provided (17). The increase of acids in this study around day 6–8 (see Figure 4) is in accordance with the findings of Stadler and others (26). However, the comparison of results from different studies remains challenging because of the differences in the experimental setup, the instrumentation, and the data treatment. Nevertheless, the finding of similar trends among decomposition studies conducted in various geographic location and different climate zones is of high importance to the field because it confirms the hypothesis that there are consistencies in the evolution of the VOC profile independent from climate. In addition, further investigating local weather conditions at the time of the trial may assist in understanding differences between regions.
FIGURE 2: GC×GC contour plot from (a) a decomposition odor sample of the fresh stage, and (b) the active decay stage of decomposition. Both are generated using flame ionization detection (FID).
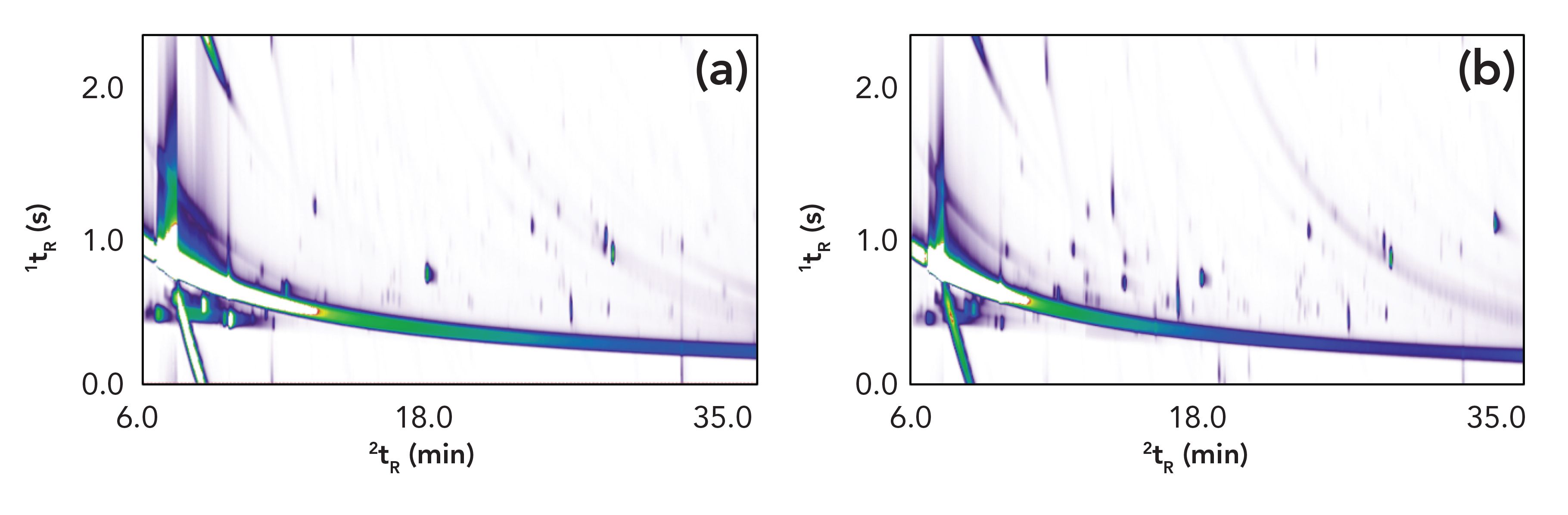
FIGURE 3: Temporal changes of all major chemical classes during the period of the trial.
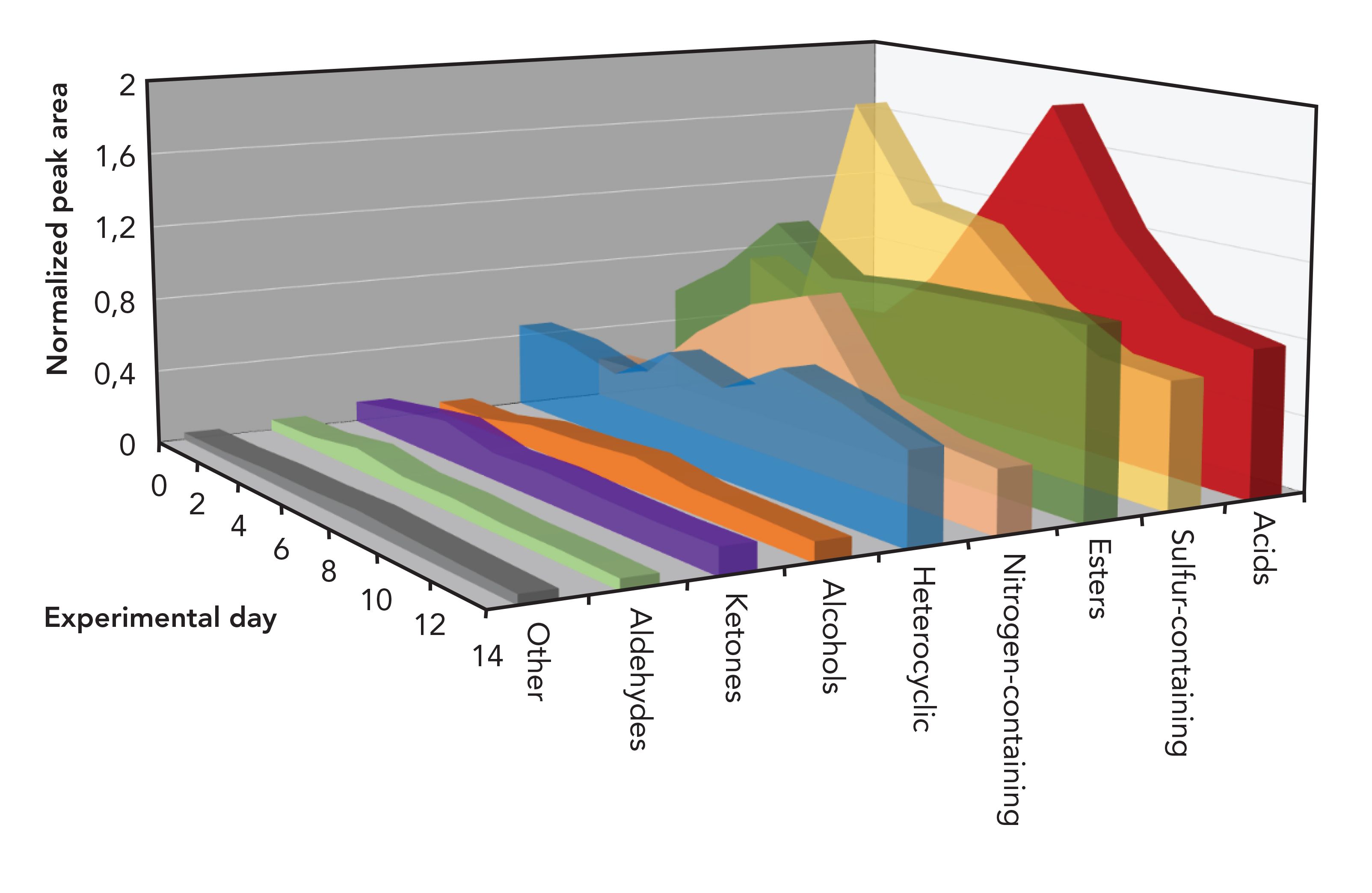
FIGURE 4: Temporal change of (a) acids, (b) sulfur-, and (c) nitrogen-containing compounds in samples and controls during the period of trial.
Decomposition Stages
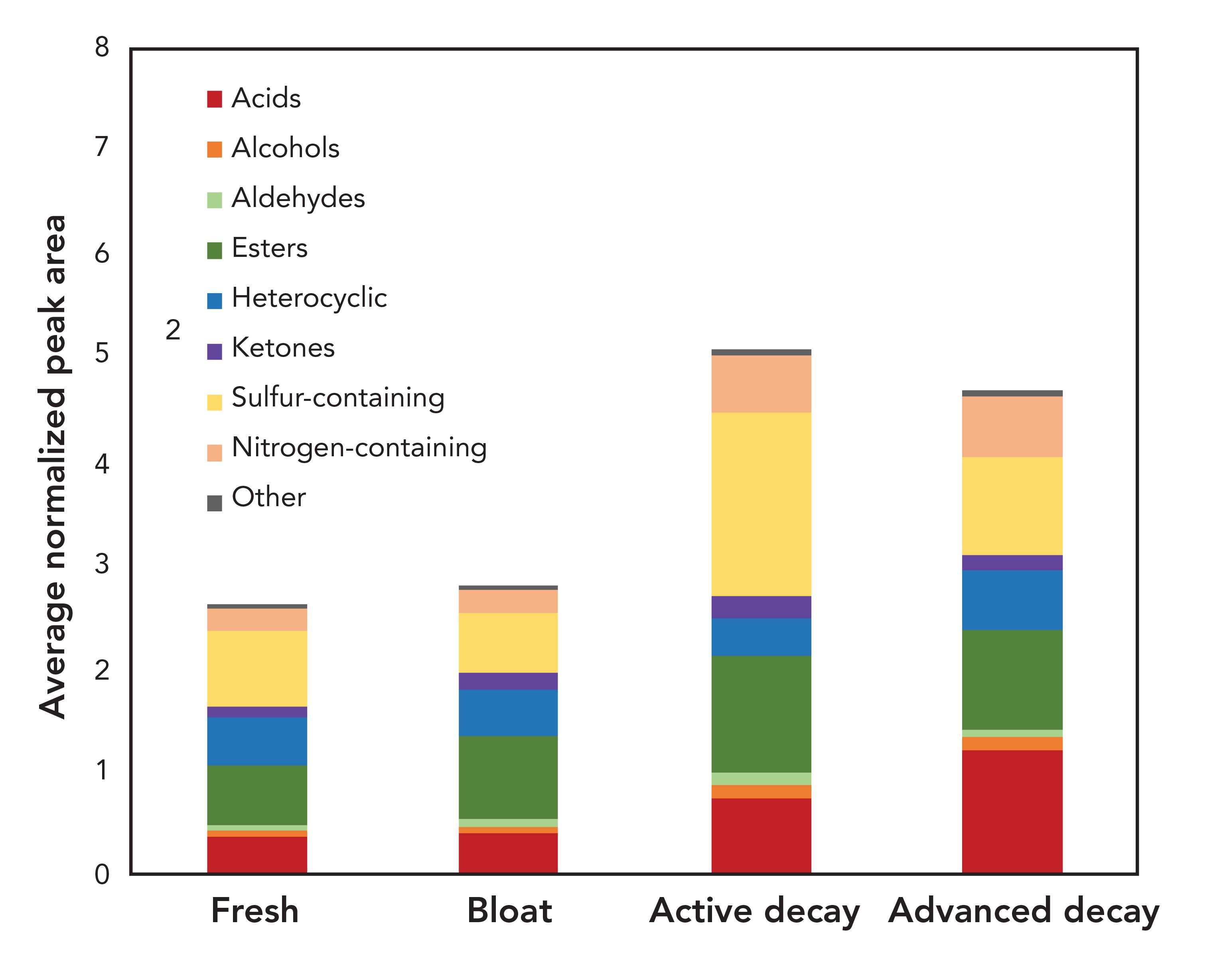
The three pig carcasses reached advanced decomposition within 14 d of the trial. Figure 5 displays the distribution of chemical classes according to decomposition stage. Fewer VOCs were detected during the fresh and the bloat stages. The highest number of postmortem VOCs was detected during the active decay stage and advanced decay stage. The primary compound classes contributing to the increase in odor during the active decay stage were acids, esters, and sulfur-containing compounds. The proportion of acids became even more prominent during the advanced decay stage. These observations are in agreement with what Dekeirsschieter and others reported, which was that a similar increase of VOCs was observable during the active decay stage and the advanced decay stage when investigating decomposition VOCs from pig remains in Belgium (21). These findings indicate that some trends can be associated with the decomposition stage, independent from the geographical location. Again, a detailed comparison is difficult because of differences in experimental parameters and data format reported in publications. Thus, the observation of comparable trends demonstrates the portion of the decomposition odor profile that transcends these differences in analytical parameters.
FIGURE 5: Chemical distribution of decomposition volatile organic compound profiles during the fresh, bloat, active decay, and advanced decay stages.
It is important to note that decomposition VOCs have been proposed as PMI estimation tools in the past. However, the majority of studies that comment on temporal trends for PMI estimation are primarily focused on using VOCs as a tool for differentiating between stages of decomposition that can be visually observed. To use specific decomposition VOCs as biochemical markers for postmortem interval, a much deeper understanding of differences in odor production under varied circumstances is required. As further studies are performed on decomposition odor, the possibility of using decomposition VOCs as biochemical markers becomes a stronger possibility, and could provide a valuable tool to operationalize odor analysis for death investigation purposes.
Field Design
Besides some major differences between humans and pigs, in the field of decomposition odor many research groups have used pig carcasses because they are considered to be adequate analogs (31) and because of the similarities between pigs and humans regarding the body composition, the gut flora, and the lack of heavy fur (32). It was only recently that the first full longitudinal field trial was performed comparing the odor from humans and pig carcasses placed in the same field environment at the same time (33). The use of human analogs mainly occurs because of two reasons: First, the use of pig carcasses reduces legal, ethical, logistical, and economical obstacles; second, and more importantly for this study, it allows for very similar carcass replicates, and it controls confounding factors that are especially important in the phase of method development and comparison. This study used pig carcasses to demonstrate the comparability of the instrumentation with previous studies. In the experimental design, an attempt was made to control for as many internal or external variables to ensure adequate biological replicates. However, validation studies with human remains are still desirable to support the hypothesis that the results for pig and human remains are comparable.
Instrument Configuration
A variety of different instruments and experimental settings have been reported for the analysis of decomposition VOCs. The most popular configuration for GC×GC analysis uses a thermal modulator and a time-of-flight (TOF) mass spectrometer for the detection and identification of analytes. In this study, an RFF flow modulator was retrofitted onto a pre-existing 1D-GC instrument and equipped with dual channel detection using a qMS instrument and a FID. This is a cost-effective alternative with the realistic potential for the adoption of GC×GC in routine forensic analysis, because laboratories are being provided with the potential to convert their pre-existing instruments into GC×GC instruments. However, there are currently no validated methods for the analysis of decomposition VOCs. Importantly, no studies have been performed in different laboratories to demonstrate inter-laboratory repeatability. Additionally, there are no reference standards for decomposition odor for researchers to use in comparing results between laboratories. Therefore, a direct comparison of this new configuration remains challenging. In addition, differences between the results presented herein and those from other studies in literature may not be based solely on the physical aspect of the instrumentation being used, but also on the way the produced data were processed. For advancement in our knowledge of decomposition odor, it would be valuable to take these points under consideration for future work. The lack of standardization across the field can be a hindrance to using decomposition odor as a valuable forensic tool.
Conclusion
In this study, the decomposition VOC profile on Oahu, Hawaii, using a retrofitted GC instrument with a RFF flow modulator and equipped with dual-channel detection using a qMS instrument and a FID, was established while taking into account environmental variables. Temporal trends were observed for acids, sulfur-containing compounds, esters, and nitrogen-containing compounds. Acids increased in the beginning of the trial, reaching their highest abundance around day 6–8 and representing a significant part of the advanced decay VOC profile. Sulfur-containing compounds increased even more rapidly in the beginning (day 2); combined with esters, they greatly contributed to the active decay VOC profile. Nitrogen-containing compounds rose to a maximum abundance around day 8 and showed an increased presence in the active and the advanced decay VOC profile.
Even though it is difficult to compare the results among different studies because of the use of different methods and instrumentation, trends in the evolution of some major chemical classes were consistent with results from studies conducted in other climate zones. Overall, this study demonstrates the robustness of the technique and, in addition, it indicates that identifying the major VOCs consistently produced by decomposition is possible in various geographical locations and forensic scenarios. However, to provide more comparable data, some of the variables between studies need to be standardized; eventually, this would allow inter-laboratory comparison studies to be performed.
References
(1) L.M. Dubois, P.H. Stefanuto, L. Heudt, J.F. Focant, and K.A. Perrault, Forensic Chem. 8, 11–20 (2018).
(2) S. Paczkowski, S. Nicke, H. Ziegenhagen, and S. Schütz, J. Forensic Sci. 60, S130–137 (2015).
(3) K. D. Nizio, M. Ueland, B. H. Stuart, and S. L. Forbes, Forensic Chem. 5, 33–45 (2017).
(4) M. Statheropoulos, C. Spiliopoulou, and A. Agapiou, Forensic Sci. Int. 153(2–3), 147–155 (2005).
(5) E.M. Hoffman, A.M. Curran, N. Dulgerian, R. A. Stockham, and B.A. Eckenrode, Forensic Sci. Int. 186(1–3), 6–13 (2009).
(6) F. Verheggen et al., Bioscience 67(7), 600–613 (2017).
(7) M.A. Iqbal, K.D. Nizio, M. Ueland, and S.L. Forbes, TrAC Trends Anal. Chem. 91, 112–124 (2017).
(8) L.M. Dubois, S. Aczon, J.-F. Focant, and K.A. Perrault, Anal. Chem. 92(14), 10091–10098 (2020).doi:10.1021/acs.analchem.0c01926
(9) K.A. Perrault, K.D. Nizio, and S.L. Forbes, Chromatographia 78(15–16), 1057–1070 (2015).
(10) M.C. Peel, B.L. Finlayon, and T.A. Mcmahon, Hydrol. Earth Syst. Sci. 11, 1633–1644 (2007).
(11) H.N. LeBlanc, K.A. Perrault, and J. Ly, Util. Arthropods Leg. Investig., 507–519 (2020). https://www.taylorfrancis.com/chapters/ edit/10.4324/9781351163767-24/role-decomposition-volatile-organic-compounds- chemical-ecology-h%C3%A9l%C3%A8neleblanc-katelynn-perrault-julie-ly
(12) S. Stadler et al., J. Chromatogr. A 1255, 202–206 (2012).
(13) P.-H. Stefanuto et al., Anal. Bioanal. Chem. 407(16), 4767–4778 (2015).
(14) J.F. Focantetal, Chem Bull Kazakh Natl Univ 4(72), 177–186 (2013).
(15) A.A. Vass et al., J. Forensic Sci. 49(4), 1–10 (2004).
(16) A.A. Vass et al., J. Forensic Sci. 53(2), 384–391 (2008).
(17) S.L. Forbes and K.A. Perrault, PLoS One 9(4), e95107 (2014).
(18) K.A. Perrault, T. Rai, B.H. Stuart, and S.L. Forbes, Anal. Methods 7(2), 690–698 (2015).
(19) K.A. Perrault et al., J. Sep. Sci. 38(1),73–80 (2015).
(20) P. Armstrong, K.D. Nizio, K.A. Perrault, and S.L. Forbes, Heliyon 2(2), e00070 (2016).
(21) J. Dekeirsschieter, P.-H. Stefanuto, C. Brasseur, E. Haubruge, and J.-F. Focant, PLoS One 7(6), e39005 (2012).
(22) E. Rosier et al., PLoS One 10(9), e0137341 (2015).
(23) C. Brasseur et al., J. Chromatogr. A 1255, 163–170 (2012).
(24) A. Agapiou et al., Anal. Chim. Acta 883, 99–108 (2015).
(25) M. Statheropoulos et al., Forensic Sci. Int. 210(1–3), 154–63 (2011).
(26) S. Stadler, P.-H. Stefanuto, M. Brokl, S.L. Forbes, and J.-F. Focant, Anal. Chem. 85(2), 998–1005 (2013).
(27) S. Stadler, J.-P. Desaulniers, and S.L. Forbes, Int. J. Legal Med. 129(3), 641–650 (2015).
(28) L.P. Chun, M.J. Miguel, E.N. Junkins, S.L. Forbes, and D.O. Carter, Sci. Justice 55(6), 394–401 (2015).
(29) J.M. Byrne, L.M. Dubois, J.D. Baker, J.F. Focant, and K.A. Perrault, MethodsX 7, (2020).
(30) M.A. Iqbal, K.D. Nizio, M. Ueland, and S.L. Forbes, Trends Anal. Chem. J. 91, 112–124 (2017). doi:10.1016/j.trac.2017.04.009
(31) K.G. Schoenly, N.H. Haskell, D.K. Mills, and C. Biemendi, Am. Biol. Teach. 68(7), 402–410 (2006).
(32) S. Matuszewski et al., Int. J. Legal Med. 134, 793–810 (2019).
(33) Z. Knobel, M. Ueland, K. D. Nizio, D. Patel, and S. Forbes, Aust. J. Forensic Sci. 51(5), 557–572 (2018).
Lena M. Dubois and Jean-François Focant are with the Molecular Systems Department and Organic & Biological Analytical Chemistry Group at the University of Liège in Liège, Belgium. David O. Carter is with the Laboratory of Forensic Taphonomy within the Forensic Sciences Unit at Chaminade University of Honolulu in Honolulu, Hawaii. Carlos Gutierrez is with the Forensic Sciences Unit at Chaminade University of Honolulu in Honolulu, Hawaii. Julianne M. Byrne and Katelynn A. Perrault are with the Laboratory of Forensic and Bioanalytical Chemistry within the Forensic Sciences Unit at Chaminade University of Honolulu, in Honolulu, Hawaii. Direct correspondence to: katelynn.perrault@chaminade.edu

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








