Improving Protein Separations with Mixed-Mode Chromatography
LCGC North America
Guest authors show how mixed modes can be used successfully in the optimization of protein purification, and discuss how various experimental parameters can be used to regulate the binding of proteins to mixed-mode sorbents.
Because proteins are polyions with both hydrophobic and hydrophilic surfaces in distinct spatial arrangements, each protein presents unique purification challenges. Although protein interactions with chromatography sorbents generally are considered in terms of single modes such as ionic or hydrophobic interactions, in fact, protein chromatography often involves multiple modes of interaction, some of which are unintended "secondary" interactions with the sorbent bead or spacer–linker structure carrying the nominal ligand. In contrast to unintended interactions, mixed-mode protein chromatography uses a sorbent intentionally functionalized with ligands capable of multiple modes of interaction to effect a protein separation process, including binding, washing, and elution. The advantages of mixing modes deserve much wider recognition. Mixed-mode chromatography can enhance selectivity beyond that of chromatography with the same single modes performed separately, can reduce the number of column steps needed for protein purification, and indeed, sometimes solves protein purification problems that are otherwise intractable.

Ronald E. Majors
These multiple modes can include ion exchange, hydrophobic, affinity, and size exclusion, as well as hydrogen bonding, π–π and thiophilic interactions, as illustrated by the imaginary multimodal ligand shown in Figure 1. In viewing the process of mixed-mode chromatography, two considerations are especially important. First, the distinct and fixed spatial array of structural elements comprising the mixed-mode ligand create a pseudoaffinity sorbent that will tend to bind to complementary structures on the protein. In contrast to the usual view of an affinity ligand binding to a specific site, a priori, for a mixed-mode ligand, there need be no known specific site. Indeed, screening various mixed-mode sorbents against a particular protein or feedstock constitutes a search for sites on the target protein (or impurities) that will provide useful affinity and selectivity. Second, there is an inherent orthogonality to the mixed-mode chromatography process, as multiple types of chromatography occur simultaneously. However, interactions between modes mean the chromatography processes are not independent. Thus, for example, in a mixed-mode ligand containing hydrophobic and ionic elements, increasing ionic strength will disrupt ionic bonds but the increasing salt concentration will promote hydrophobic adsorption.
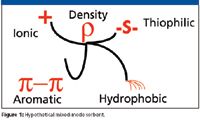
Figure 1
Mixed Mode is More Common Than Recognized
Chromatographers should not be surprised to find they already practice mixed-mode chromatography. It is well known that base matrix, linker, and linker chemistry influence the chromatographic process through interactions that are generally different than the primary mode. These interactions constitute a hidden form of mixed-mode chromatography. The hydrophobic properties of the linker used in the weak ion-exchange sorbent DEAE-Sephadex are an instance of a less obvious mixed-mode sorbent. Affinity ligands virtually always involve mixed modes of interactions and bind to a specific site on the protein. For example, Cibachrome Blue, a hydrophobic dye containing sulfonates, amines, and benzyl groups, binds to a nucleotide pocket on proteins. A variety of "custom ligands," such as the dye-based mixed-mode sorbents offered by Prometics Life Sciences (Cambridge, U.K.), interact via multiple modes. Protein-based affinity ligands also interact with their targets through a plethora of interactions. For example, the well-known antibody affinity ligand protein A binds to the Fc region of antibodies through hydrophobic, hydrogen bonding, and ionic interactions. Elution from protein A is effected by lowering the pH so that a highly conserved histidine on the antibody is protonated, setting up a charge repulsion with nearby cationic sites on protein A (1).
Other common examples of mixed-mode sorbents include many of the "aqueous" reversed-phase columns that incorporate ionic groups into their hydrophobic phase to promote wettability. Solid-phase extraction sorbents also frequently operate using mixed-mode interactions, but the pore sizes of these sorbents are usually too restrictive for protein chromatography. Recently, however, analytical high performance liquid chromatography (HPLC) wide-pore columns containing ionic and hydrophobic ligands have been introduced (for example, ProMix from SIELC, Prospect Heights, Illinois) that are suitable for protein analysis.
Fewer Steps, Increased Selectivity
By effectively combining orthogonal chromatography techniques into a single-sorbent, multimodal chromatography can reduce the total number of column steps needed in a purification process. Also, because the mixed-mode elements are present in a single ligand, there is a fixed spatial relationship between them that adds a facet of pseudoaffinity binding and therefore selectivity. Binding is more likely to occur on patches on the protein surface that are complementary to each mode and with matching spatial configurations. This is one reason why mixed-mode sorbents offer selectivity beyond that which can be achieved by simple mixing of, for instance, hydrophobic interaction chromatography (HIC) and anion-exchange sorbents. It seems likely that the pseudoaffinity properties created by the fixed spatial relationship of the mixed-mode ligand elements contributes significantly to the selectivity of mixed-mode sorbents.
Binding and elution to mixed-mode sorbents are controlled by the parameters relevant to each mode, that is, lyotropic salt for hydrophobic interactions and ionic strength for ionic interactions. There are opportunities for optimizing each, such as salt type, buffer type and strength, additives, and pH. Furthermore, the interactions between these parameters permit fine-tuning of the chromatography process. As the number of parameter combinations increases, the design of experiment (DOE) approach is worth considering for optimizing the purification process. However, even without the use of DOE, one can often optimize separation by making intelligent guesses aided by an understanding of relevant parameters.
Hydroxyapatite is a Mixed-Mode Sorbent
Hydroxyapatite is one of the oldest forms of chromatographic media. This sorbent is unique in that it is a crystalline structure of phosphate and calcium in which the matrix and the active sites are the same. The phosphate sites provide for ionic interactions and are controlled by ionic strength, while the calcium sites provide for metal affinity binding, which is disrupted by increasing the phosphate concentration. The crystalline structure of hydroxyapatite gives a fixed spatial relationship between the active sites, which can result in pseudoaffinity interactions that impart unique selectivity to the separation process not possible with other modes of chromatography (2,3).
Mixed-Mode Hydrophobic Ion-Exchange Ligands
Figure 2 shows the structure of ligands used on several recently introduced mixed-mode sorbents intended for protein purification. These ligands incorporate both hydrophobic and ionic elements. Each factor applies to mixed mode just as it does for single mode chromatography. Thus, pH, salt type, and ionic strength, as well as buffer type and strength, all come into play for ion exchange; for hydrophobic interactions, salt type and concentration, and also additives are important. What is different in mixed mode is that the various binding and elution factors are not independent and can work in opposing ways. For example, increasing the ionic strength for ion-exchange elution will drive hydrophobic binding. Because of these dependent interactions, buffer conditions must be optimized carefully at each step of binding, washing, and elution to find the right balance. Very high selectivity can result when the right balance is found.
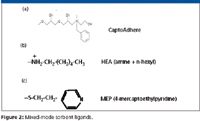
Figure 2
Hydrophobic, Anionic Ligand with Hydrogen-Bonding
CaptoAdhere (Figure 2a (GE Healthcare, Piscataway, New Jersey) is intended to be used to further purify antibodies eluted from protein A columns. The CaptoAdhere ligand features hydrogen bonding, a quaternary amine, and a phenyl group. Antibodies, which are generally basic, do not bind to the ligand due to charge repulsion, whereas various acidic and hydrophobic contaminants (for example, protein A leachate, nucleic acids, endotoxin, and many host cell proteins) are adsorbed. In a flow-through mode, one needs only to be concerned with maximizing recovery and binding of contaminants, so there are no washing and elution steps. The purity of the antibody found in the flow-through fractions can be increased by optimizing the pH, the buffer and buffer strength, and the salt species and ionic strength. Not surprisingly, increasing pH increases product purity, as more of the negative contaminants are adsorbed to the resin. However, product yield decreases. The salt concentration is set optimally by balancing the effects of low salt, which promote binding via ionic interactions, and high salt, which drives hydrophobic binding but disrupts ionic interactions. DOE has been used with CaptoAdhere to derive the optimum balance of pH, ionic strength, and lyotropic salt (4).
Mixed-Mode pH-Controllable Sorbents
MEP HyperCel (Pall Life Sciences, New York, New York) contains a 4-mercaptoethyl pyridine ligand (Figure 2b). The pyridine ring is uncharged at neutral and basic pH. As the pH decreases, the pyridine nitrogen acquires a proton to become positively charged, turning the resin into a mixed-mode sorbent. MEP is therefore a "pH-controllable" mixed-mode ligand, a property that has been termed "hydrophobic charge induction chromatography" (5).
The MEP ligand has an affinity for a broad range of immunoglobulins (IgA, IgE, IgM, IgY) and for IgGs that are retained poorly on protein-A sorbents (for example, murine and rat IgG). MEP HyperCel can be used to capture antibodies directly from supernatant in a manner similar to protein A but in the absence of lyotropic salts. The hydrophobic nature of the adsorption is seen in the decreased binding capacity of antibodies with additives such as ethylene glycol (6).
Elution of antibodies from MEP occurs by lowering the pH so that the pyridine becomes positively charged and the protein is released by charge repulsion (Figure 3). Many of the contaminants remain bound to the resin as the ligand acquires cationic characteristics. As the pH is lowered, the pyridine ring acquires an increasingly positive charge which causes basic species to bind less tightly while acidic species bind more tightly. Washing the sorbent at the lowest pH at which the antibody remains bound removes loosely adsorbed contaminants. Optimal purity usually is obtained when the pH is lowered to where the antibody just desorbs, so that more of the contaminants remain bound. Fine-tuning separation is performed by adjusting the salt concentration. Because increasing the salt concentration promotes hydrophobic binding, proteins will tend to elute at a lower pH as the conductivity of the buffer is increased, as shown by Broschetti (6) for antibodies.
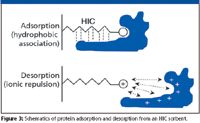
Figure 3
Unlike conventional HIC, some workers report that increasing the salt concentration reduces the dynamic binding capacity of the resin for antibody (7). Others report that dynamic binding capacity at near neutral pH is largely independent of salt concentration over a broad range (for example, 50 mM to 1 M sodium chloride) (8,9). That said, moderate increase in the conductivity of the elution buffer (for example, addition of sodium chloride to vary conductivity over the range of 5 to 50 mS/cm) can increase binding affinity (6). Such increased binding affinity would be expected for a hydrophobic interaction sorbent. Significantly, such increased binding affinity can be overcome by reducing the pH of the elution buffer and increasing the magnitude of electrostatic charge repulsion effects. As a practical matter, this behavior demonstrates that hydrophobic charge induction chromatography (HCIC) can be optimized based upon both pH and ionic strength. Optimizing ionic strength can be particularly advantageous during post-load wash steps. Here, reduced conductivity can be used to prompt elution of weakly bound impurities. This approach was used to purify a botulinum neurotoxin fragment, a protein of interest for its ability to confer protective immunity to native botulinum toxin (10). Optimizing post-load wash steps also has been employed to purify monoclonal antibodies (9).
MEP also is well suited for purifying recombinant proteins. However, unlike antibodies, recombinant proteins generally will need salt to promote binding. The pKa of the pyridine is 4.8, so the MEP ligand is uncharged at neutral pH and so at neutral and alkaline pH, binding to the MEP ligand is promoted by lyotropic salts in a manner similar to other HIC sorbents. However, due to the high ligand density of the MEP HyperCel sorbent, binding will often occur at lower salt concentrations than those used with most HIC sorbents such as phenyl. Desorption from MEP can be controlled by decreasing the lyotropic salt and by adjusting pH so the charge on the protein is the same as the charge on the resin. In general, as pH decreases, more basic proteins will be eluted first, and acidic proteins will be eluted at progressively lower pH values. Similarly, other things being equal, more hydrophilic proteins will be eluted earlier than more hydrophobic ones (Figure 4).
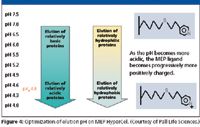
Figure 4
While recovery is often poor from very hydrophobic resins, the charge repulsion on protonation of the MEP ligand causes the protein to be pushed off the resin when the pH is below the protein isoelectric point. As mentioned earlier, raising the salt concentration increases hydrophobic binding and decreases ionic interactions. Thus, a balance of ionic strength, lyotropic salt, and pH is used to optimize selectivity. Susceptibility to charge repulsion and lyotropic salts affect species differently, which can be used to improve separation. This approach has used the mixed-mode properties of MEP advantageously to separate antibody dimers and aggregates from monomeric antibody (11), as dimers and aggregates are more hydrophobic than monomers. Monomeric antibodies will tend to be eluted at a higher pH than aggregates.
If protein binding to MEP was purely hydrophobic, one would expect that a recombinant protein would be eluted as salt concentration decreases. However, we have found that, once bound, the target protein frequently remained adsorbed to MEP even as the lyotropic salt concentration decreased. Hysterisis effects are well-known in HIC but can be more extreme in mixed-mode chromatography, likely due to pseudoaffinity binding. Also in common with hydrophobic chromatography, but more extreme, is that binding conditions can affect conditions for elution and recovery. Thus, binding in excessively high salt might mean that more rigorous conditions (for example, a lower than expected pH) are needed for elution. This can improve separation if, for example, it can be used to promote retention of one species over another.
These principles are illustrated in the purification of a recombinant protein using MEP HyperCel, as shown in Figure 5. Binding conditions were selected by determining the lowest lyotropic salt concentration and pH that gave high-capacity binding of the target protein, in this case 1 M ammonium sulfate at pH 8. The column was then washed with low salt buffer at the same pH. The target protein was retained. The column was then washed with a buffer at the lowest pH that retained binding. We then selected the highest pH that caused desorption of the target protein. During process development, we found that minor adjustments needed to be made to the salt concentration and pH to promote retention of contaminants. Contaminants eluted at the tail of the pH 6 elution peak and at pH 4.
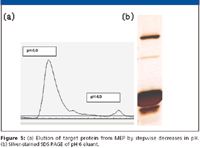
Figure 5
Mixed-Mode Charge-Assisted Hydrophobic Binding
HEA HyperCel (Pall Life Sciences) (Figure 2c) consists of a hexyl ligand containing a secondary amine, and so is cationic over a wide pH range. As such, it acts as both a hydrophobic interaction resin and an anionic exchange sorbent. HEA is therefore capable of binding acidic proteins and proteins with a moderately high isoelectric point, at low ionic strength. HIC binding to HEA still predominates, as indicated by the increase in binding capacity with temperature (12). Furthermore, lyotropic salts markedly increase binding capacity, despite increased ionic strength. In view of the predominance of hydrophobic binding to these mixed-mode ligands, binding to ligands like HEA can be viewed as "charge-assisted hydrophobic binding."
Elution from HEA is promoted by reducing pH, which increases the positive charge on the protein, resulting in charge repulsion. In our experience, for acidic proteins pH generally needs to be reduced well below the pI to promote elution, even though one would expect good elution due to charge repulsion. This likely is due to the dominance of hydrophobic binding. We speculate that the ligand tends to bind in a hydrophobic patch adjacent to a negative charge, forming a salt bridge essentially as a pseudoaffinity binding site. Due to this pseudoaffinity binding, salt bridges can remain intact even as the pH is lowered below the pI, preventing elution. Increasing the ionic strength would break the salt bridges but also promote hydrophobic binding, making protein elution more difficult. One solution is to use additives such as propylene glycol to reduce hydrophobic interactions.
Semiclassical HIC
Interestingly, in the presence of lyotropic salts, even very basic proteins will bind to HEA HyperCel, despite charge repulsion. The ability of lyotropic salts to promote binding to mixed-mode sorbents despite charge repulsion allows for a unique form of HIC that we term "semiclassical HIC." The crude mixture is bound to the sorbent at a high pH, with salt if necessary. The buffer pH is then changed to one in which charge repulsion usually would cause elution, but sufficient salt is included to maintain binding. A descending salt gradient is then run to elute the target protein, as is used in normal HIC. This technique differs from the usual HIC protocol in that the driving force for elution is charge repulsion, as well as the reduction in lyotropic salt. One can adjust the process to improve selectivity by lowering or raising the pH.
Contaminant Removal Applications using Mixed Mode
Mixed-mode chromatography resins that combine hydrophobic and anion exchange properties also are useful for removing contaminants that are both acidic and hydrophobic, such as lipopolysaccharide (LPS). LPS levels need to be very low for therapeutic proteins, yet LPS reduction can be difficult, particularly for basic proteins. LPS forms micelles that do not enter the pores of conventional chromatography sorbents. Consequently, anion-exchange resins are often ineffective at removing LPS when used in a flow-through mode. In contrast, if the mixed-mode resin binds the protein, micellular LPS flows through the sorbent. The protein is then eluted by lowering the pH to induce charge repulsion, while the residual LPS that does bind to the resin remains strongly bound to the hydrophobic and cationic ligand on the resin. Thus, conditions that promote desorption of the protein promote retention of the LPS.
Conclusions
Mixed-mode chromatography offers unique approaches to improving selectivity. Mixed-mode ligands are enhanced by the fixed spatial relationship between their elements, which likely results in pseudoaffinity binding. Moreover, because mixed modes offer the ability to modulate adsorption properties differentially, their selectivity is enhanced beyond that of a series of chromatography steps involving the same single modes. Furthermore, by combining multiple modes of chromatography in a single step, the total number of column steps needed for a purification process is often reduced.
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com
Andrew Lees, Ph.D.,is Scientific Director at Fina BioSolutions LLC in Rockville, MD. Fina Biosolutions engages in downstream bioprocessing. In addition, the company is active in the development of protein-polysaccharide conjugate vaccines. e-mail:ALees@FinaBio.com
Marty Burkhardt is Vice President of Manufacturing at AFG Biosolutions, Inc. in Gaithersburg MD. e-mail: marty.burkhardt@afgbio.com
References
(1) P.S. Gagnon, Purification Tools for Monoclonal Antibodies (Validated Biosystems, Inc., Tucson, Arizona, 1996), pp. 155–190.
(2) T. Kawasaki, M. Niikura, and Y. Kobayashi, J. Chrom. 515, 125–148 (1990).
(3) P.S. Gagnon, P. Ng, J. Zhen, C. Aberin, and J. He, BioProcess Int. 4, 50–60 (2006).
(4) GE Healthcare, Application Note 28-9078-89 AA.
(5) S. Burton and D. Harding, J. Chromatogr., A 814, 71–81 (1995).
(6) E. Broschetti, J. Biochem. Biophys. Meth. 49, 361–389 (2001).
(7) J. Chen, J. Tetrault, and A. Ley, J. Chromatogr., A. 1177, 272–281 (2008).
(8) L. Guerrier, P. Girot, W. Schwartz, and E. Broschetti, Bioseparation 9(4), 211–221 (2000).
(9) W. Schwartz, D. Judd, and M. Wysocki, J. Chromatogr., A 908, 251–263 (2001).
(10) G.T. Weatherly, A. Bouvier, D.D. Lydiard, J. Chapline, I. Henderson, J.L. Schrimsher, and S.R. Shepard, J. Chromatogr., A 952, 99–110 (2002).
(11) W. Schwartz, J. Jiao, J. Ford, D. Conrad, J-F Hamel, P. Santambien, L. Bradbury, and T. Robin, Bioprocessing J. (Sept/Oct) 53–62, 2004.
(12) Pall Life Sciences technical literature.

Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.











