The Impact of Neonicotinoids on Honeybee Colonies: Ultra-Sensitive and Rapid Assay of Neonicotinoids in Honey by UHPLC–MS–MS
Mikaël Levi1 and Stéphane Moreau2, 1Shimadzu France, 2Shimadzu Europa GmbH
Neonicotinoids are a class of insecticides widely used in agriculture to protect fields (corn, canola, soybeans) as well as fruits and vegetables. As contact or stomach insecticides, they are effective mostly against sucking and some chewing insects. Neonicotinoids are neuro-active insecticides and have an impact on the central nervous system of insects causing paralysis and death.
The first neonicotinoid, imidacloprid, was patented by Bayer CropScience. The systemic distribution of neonicotinoids throughout the whole plant has high efficacy against sucking insects. The long residual activity made these insecticides very popular within the global pesticide market.
Today, there are about 10 molecules classified as neonicotinoids; these include acetamiprid, clothianidin, imidacloprid, thiacloprid, thiamethoxam, dinotefuran, and nitempyram. These active components are commercialized under various brand names by international companies.
Recently, the use of these compounds has become controversial because they have been singled out as one cause of the honeybees’ Colony Collapse Disorder (CCD). Since pollination is essential for agriculture, extensive studies have been conducted to evaluate the impact of neonicotinoids on bee health.
The European Food Safety Authority (EFSA) has identified risks to bees in the use of neonicotinoids. Three types of insecticides are concerned: clothianidin, imidacloprid, and thiamethoxam. They may have acute and chronic effects on survival and development of bee colonies, their behaviour, and their larvae.
Following this, the EFSA limited the use of thiamethoxam, clothianidin, and imidacloprid. Some European countries have banned or restricted the use of neonicotinoids.
In order to better understand the effect of these compounds on bees and their contamination in pollen and honey, a highly sensitive assay method is required. For that purpose, an ultrahigh-pressure liquid chromatography tandem mass spectrometry (UHPLC–MS–MS) analysis was developed.
Materials and Methods
Standards and Reagents: All the analytical standards were provided by Sigma-Aldrich. The internal standards thiamethoxam-d3, imidacloprid-d4, and chlothianidin-d3 were purchased from SigmaâAldrich. The solvents used, including water and mobile phase additive, were of UHPLC–MS quality (Biosolve).
Sample Preparation: Compound extraction was performed using a QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method with an additional dispersive solid-phase extraction (dSPE) step.
A sample of 5 g of honey (± 1%) was weighted in a 50 mL polypropylene tube. Five microlitres of internal standard solution at 5 µg/mL of each compound in acetonitrile was added to the honey and was dried for 10 min. Ten millilitres of ultrapure water were added and the samples were homogenized by vortex mixing for 1 min. Ten millilitres of acetonitrile were then added followed by vortex mixing for 1 min.
A salts mix (4 g MgSO4, 1 g sodium citrate, 0.5 g sodium citrate sesquihydrate, 1 g NaCl; Biotage Q0020-15V) was added to the samples. After manual shaking, samples were centrifuged at 3000 g for 5 min at 10 ºC.
The supernatant (6 mL) was transferred into a 15 mL tube containing 1200 mg of MgSO4, 400 mg PSA, and 400 mg C18 (Biotage Q0050-15V). After centrifugation at 3000 g and 10 ºC for 5 min, the supernatant was transferred into inert glass vials for analysis (Shimadzu LabTotal 227-34001-01).
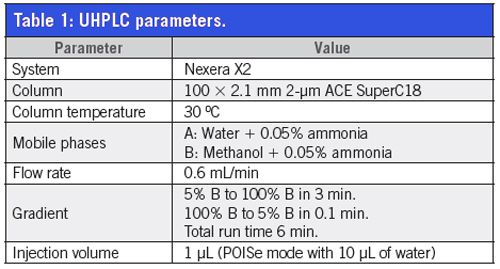
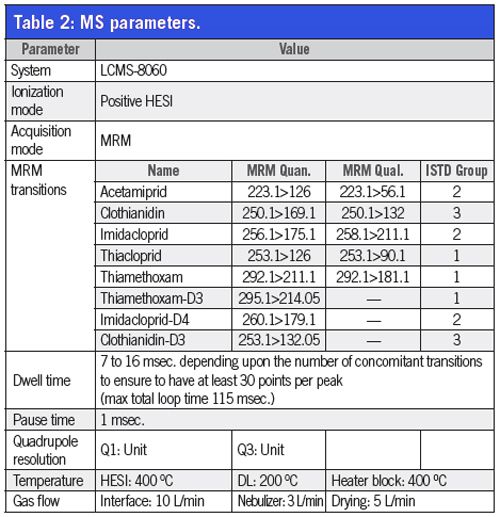
UHPLC–MS–MS Conditions: Analysis was performed using a Nexera X2 UHPLC system coupled with a LCMS-8060 triple quadrupole mass spectrometer with heated ESI in positive ionization. All parameters are described in Tables 1 and 2.
Mobile phase composition was optimized to generate the highest sensitivity. Ion source parameters (gas flows, temperatures) were also optimized using the Interface Setting Support Software (Shimadzu Corp.)
As the final extract is in an organic solvent, large injection volumes are not possible without compromising separation and peak shapes. Evaporating the extracts and reconstituting them in aqueous solvent is tedious and generates additional costs as well as the risk of losses. Therefore, a special injection mode POISe (Performance Optimized Injection Sequence) was used that enables co-injection of water with the sample to counteract the organic solvent effect.
Results
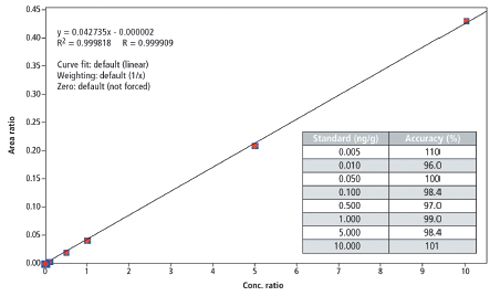
Calibration: Calibration curves were prepared in acetonitrile to obtain final concentrations ranging from 2.5 pg/mL (2.5 fg on column) to 5 ng/mL. These concentrations correspond to 5 ppt and 10 ppb in honey, respectively. A typical calibration curve is shown in Figure 1.
Figure 1: Calibration curve of clothianidin.
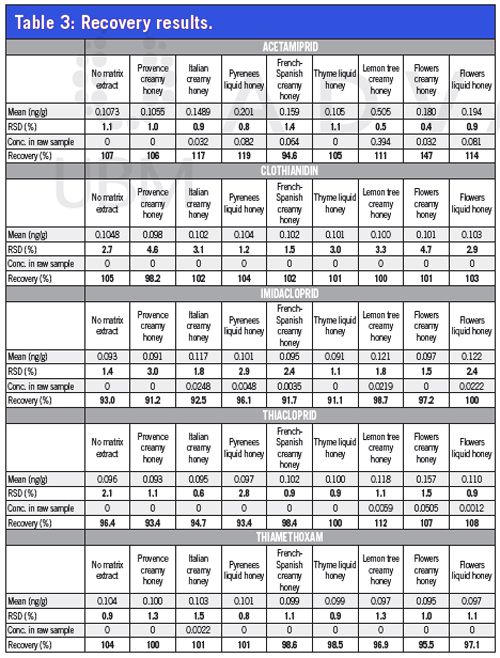
Recovery: Eight different honeys from the local supermarket were extracted with and without spike at 0.1 ppb. Each extract was injected five times to evaluate repeatability in matrix samples. A blank extract (no honey) was prepared to evaluate losses or non-specific interactions. Results are presented in Table 3.
Real Sample Analysis: The eight samples used for recovery experiments were then assayed as unknowns. Thanks to the very high sensitivity reached, even low concentrations of neonicotinoids were quantified. The detailed results are presented in Table 4.
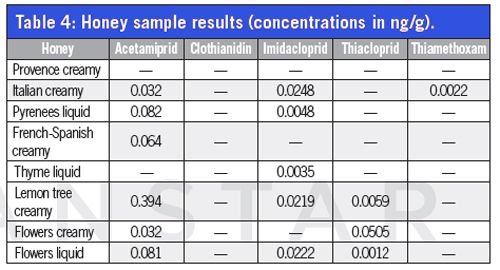
Figure 2: Calibration standard at 0.0025 pg/mL.
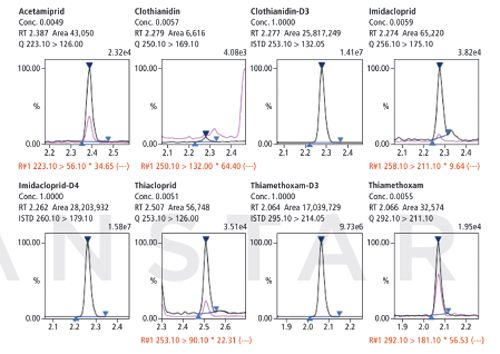
Figure 3: Lemon tree sample.
Conclusion
A method for the ultra-sensitive assay of neonicotinoids in honey was set up. Sample preparation was simple but provided excellent recoveries, regardless of the honey type. The injection mode used prevented the use of tedious evaporation/reconstitution or dilution steps. The sensitivity obtained enabled an assay in real samples at very low levels far below the regulated residue levels. This method can be a very efficient support tool to better understand the impact of neonicotinoids on honeybee colonies.
Shimadzu Europa GmbH
Albert-Hahn-Str. 6–10, D-47269 Duisburg, Germany
Tel: +49 203 76 87 0 fax +49 203 76 66 25
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Using Chromatography to Study Microplastics in Food: An Interview with Jose Bernal
December 16th 2024LCGC International sat down with Jose Bernal to discuss his latest research in using pyrolysis gas chromatography–mass spectrometry (Py-GC–MS) and other chromatographic techniques in studying microplastics in food analysis.
The Use of SPME and GC×GC in Food Analysis: An Interview with Giorgia Purcaro
December 16th 2024LCGC International sat down with Giorgia Purcaro of the University of Liege to discuss the impact that solid-phase microextraction (SPME) and comprehensive multidimensional gas chromatography (GC×GC) is having on food analysis.