Identifying Markers of Quality in Curry Powder Using GC×GC–TOF-MS
Laura McGregor, Stefan Koschinski, and Elinor Hughes, SepSolve Analytical Assessing aroma profiles with high-capacity sorptive extraction combined with GC×GC–TOF-MS and powerful data mining software.
High-capacity sorptive extraction—a sampling technique that involves using a probe to extract volatile and semi-volatile organic compounds (VOCs and SVOCs) from samples—followed by two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC–TOF-MS) and chemometric analysis was used to identify markers of quality in curry powder with high sensitivity in an automated time-saving workflow.
Herbs and spices are used in many food preparation products. For food manufacturers, generating aroma profiles from the volatile and semi‐volatile organic compounds (VOCs and SVOCs) emitted by their products is important. The profiles are used to evaluate the composition of the product to identify components causing desirable or undesirable characteristics, for quality control, to monitor changes that occur during shelf‐life, and to ensure production‐line consistency of products from batch to batch. Profiles can also help to identify differences between brands, which is of interest to manufacturers who wish to compare their product with a competitor’s.
However, the aroma profiles of spices are complex, consisting of hundreds of odor‐active compounds from a variety of chemical classes over a wide concentration span. Some compounds may have very low odor thresholds, which means that even those at trace level can have a major impact on the overall aroma. These trace compounds can be difficult to detect among more abundant components, so a highly sensitive analytical method is essential for quality assessments.
Here, we demonstrate that the sensitivity required can be achieved with the use of high‐capacity sorptive extraction with trap‐based focusing (which delivers a sharp, concentrated band of vapor to the gas chromatography [GC] sytem). We will demonstrate how this extraction, coupled with enhanced separation by two‐dimensional chromatography (GC×GC) and identification by time‐of‐flight mass spectrometry (TOF‐MS), can give greater insight into the aroma profiles of curry powder.
In addition, we will show how novel chemometric analysis can be used to compare the complex chromatograms and automatically discover the most significant differences between two curry powders of different quality.
Experimental
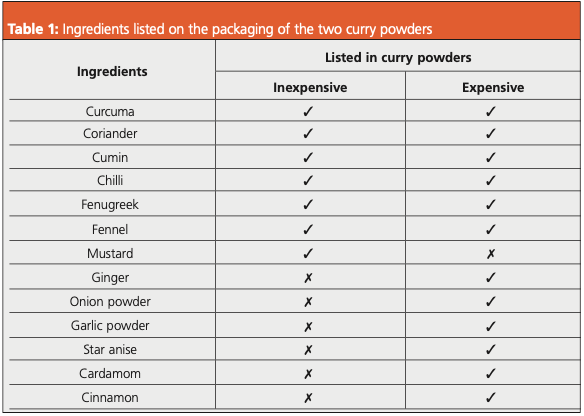
Two shop‐bought curry powders were used in this study—one value (inexpensive) brand and one premium (expensive) brand. The ingredients listed on the packaging for each curry powder are shown in Table 1. 50 mg of curry powder was weighed into a 20 mL vial, prior to the addition of 10 mL water and 2 g NaCl. Immersive sorptive extraction was then performed using an inert‐coated stainless steel HiSorb probe (Markes International) on the automated Centri extraction and enrichment platform (Markes International). Equilibration time: 5 min; temperature: 40 °C; sampling time: 30 min; agitation: 500 rpm. Prior to desorption, the probes were automatically rinsed with deionised water to remove residual matrix and dried using N2. The probes were then desorbed at 250 °C (for 15 min). GC×GC: Insight flow modulator (SepSolve Analytical); modulation period (PM): 5 s. MS: Instrument: BenchTOF‐Select (SepSolve Analytical); mass range: m/z 35–400; acquisition rate: 50 Hz. Software: Full instrument control by ChromSpace with chemometric analysis in ChromCompare+.
Results and Discussion
The high‐capacity sorptive extraction probes used in this study offer a large volume of polydimethylsiloxane (PDMS) stationary phase (65 μL), providing high sample loadings. Used in conjunction with advanced cryogen‐free focusing, this approach offers optimal sensitivity as well as the ability to fully automate sample preparation prior to desorption to the GC×GC–TOF‐MS for separation and identification of a wide range of chemical classes.
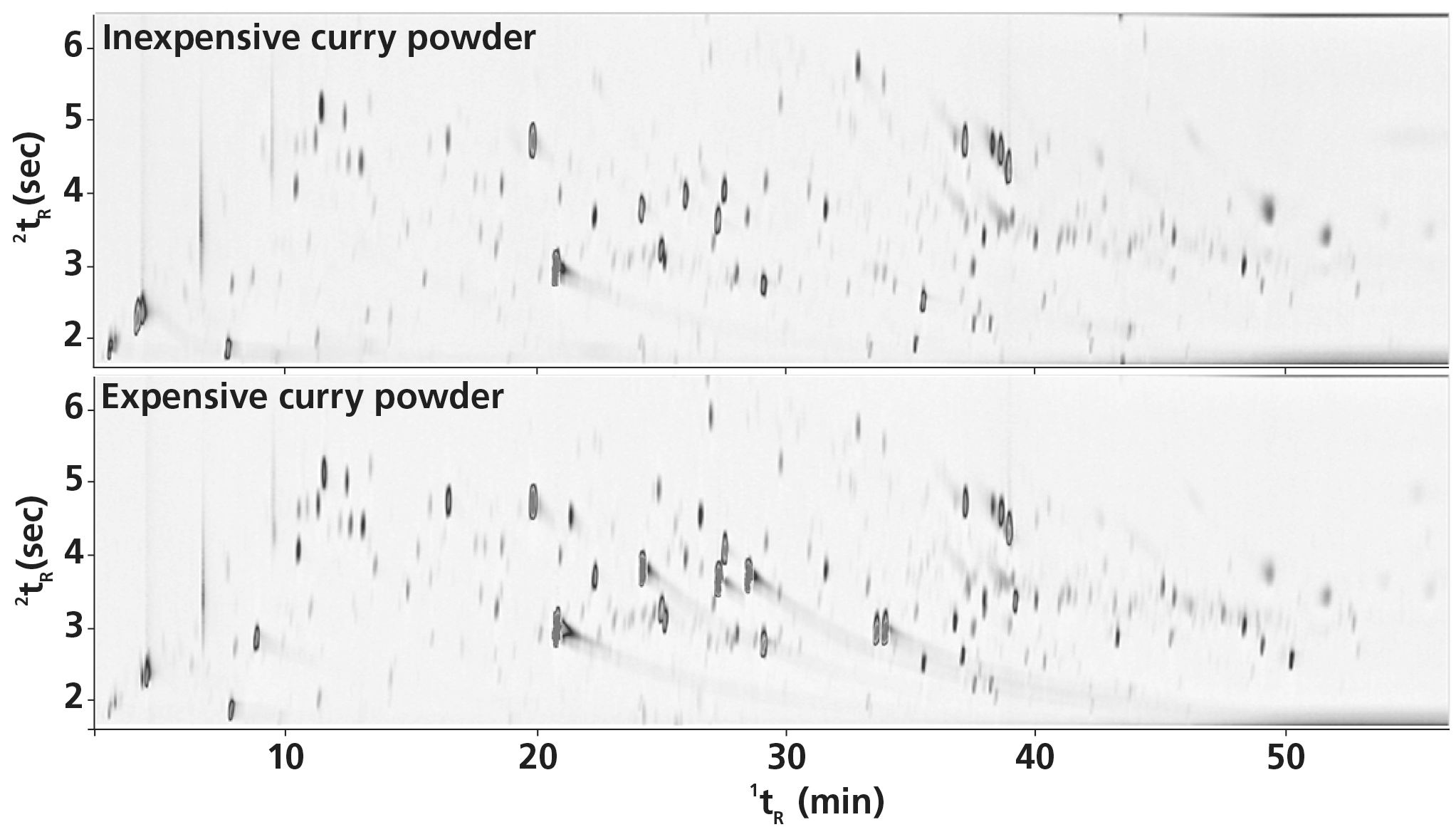
The power of using immersive sorptive extraction with GC×GC–TOF‐MS is evident in Figure 1, which shows a comparison between the aroma profiles of the inexpensive and expensive curry powders. Over 300 peaks representing a wide range of chemical classes are shown after sampling, separation, and detection. Numerous co‐elutions that would have occurred with 1D gas chromatography (GC) are avoided thanks to the additional separation in the second dimension with 2D GC×GC.
Figure 1: GC×GC–TOF‐MS colour plots for the inexpensive (top) and expensive (bottom) curry powders, sampled using immersive sorptive extraction.
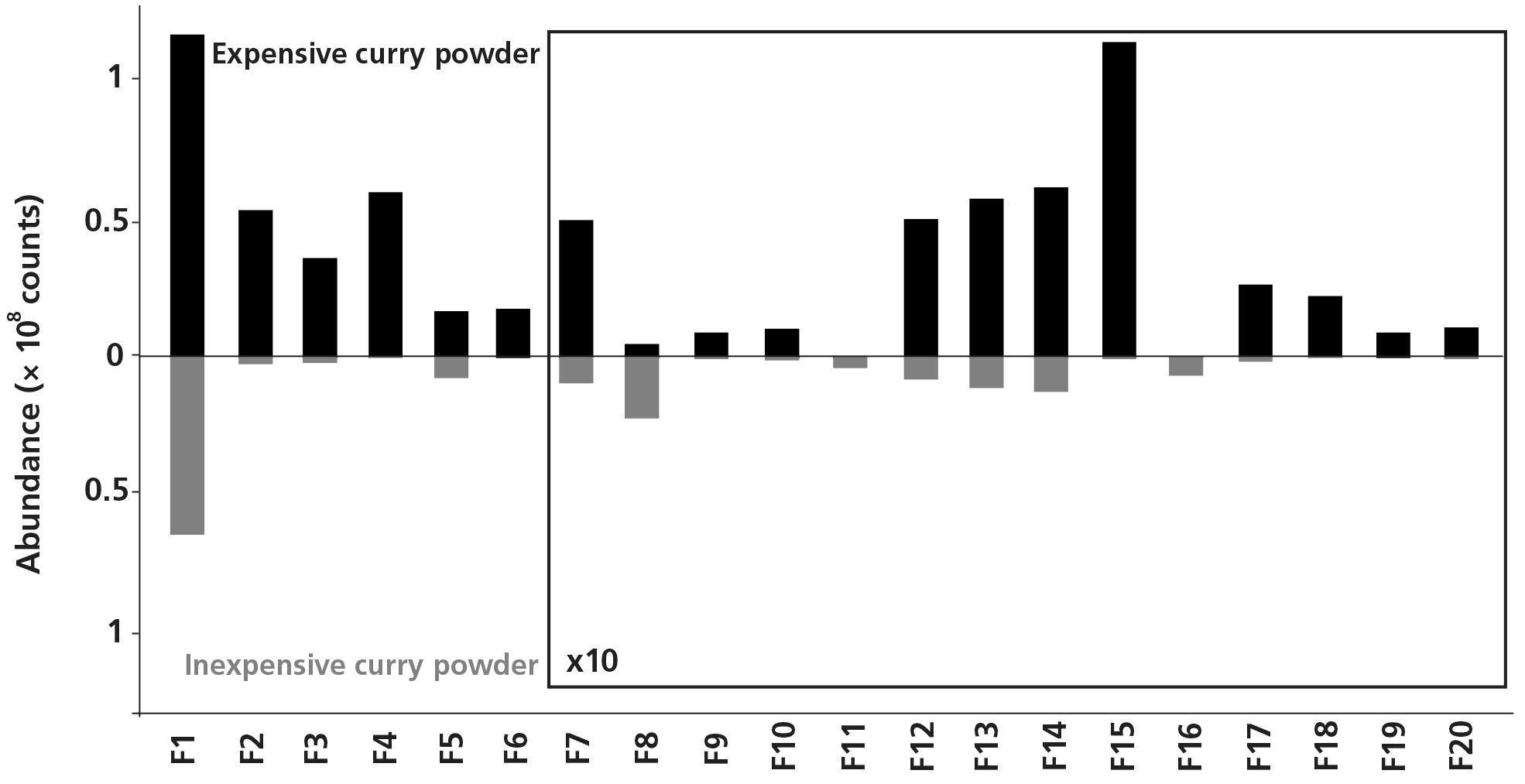
Until now, comparing such complex aroma profiles has been a major challenge in GC×GC analyses, often requiring time‐consuming manual review by an experienced analyst, with integration and identification of hundreds of peaks, many of which would ultimately be insignificant. In this study, chemometric analysis was used to tackle this challenge. The raw data were aligned and imported directly into the chemometrics platform using a fully untargeted workflow. The platform’s “Feature Discovery” tool was used to automatically reduce over 300,000 analytical features from the raw data to the top 20 most significant features, that is, those that were significantly different between the two curry powder chromatograms. These differences can be viewed easily using histogram plots (or H‐plots) as shown in Figure 2.
Figure 2: H‐plots comparing the abundances of the 20 most significant features that differ between the two curry powders. The identity and aroma attributes of each feature are provided in Table 2.
By using this approach, time‐consuming manual steps are minimized and the most significant differences can be identified quickly. The chemometrics platform automatically highlights the retention time and m/z channel where significant differences are observed, allowing fast review and identification of the important features.
As expected, many of the top 20 features are associated with the additional ingredients used in the expensive curry powder (Table 2). For example, zingerone is found in ginger (1), anethole and p-anisaldehyde have been detected in star anise (2), and cinnamaldehyde is a major constituent of cinnamon (3). Each of these components was found at elevated levels in the expensive curry powder, which illustrates how this approach can be used to identify chemical markers of quality.
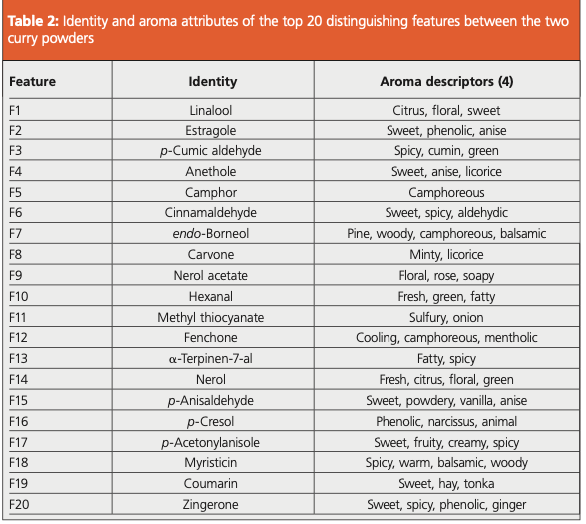
Several compounds that may have a negative impact on the aroma were identified in the inexpensive curry powder. For example, methyl thiocyanate gives a “sulfury” odor (4), which could be a result of including mustard in the ingredients.
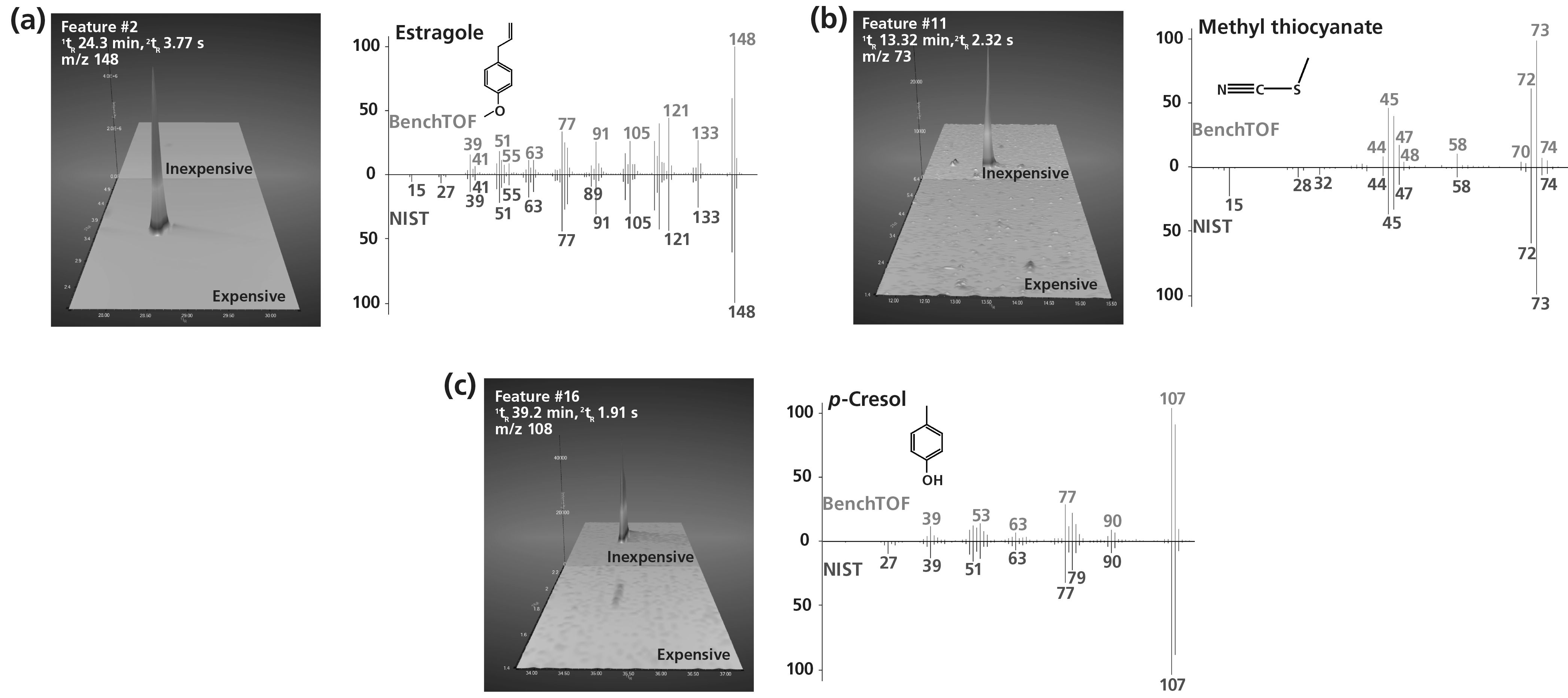
The enhanced regions of the chromatograms in Figure 3 display a selection of the top discriminating features and their confident identification by comparison of TOF mass spectra against the National Institute of Standards and Technology (NIST) mass spectral library. These differences were uncovered quickly and easily by the untargeted workflows in the chemometrics platform, even trace differences, such as for methyl thiocyanate, which had an intensity three orders of magnitude lower than the highest‐loading peaks.
Figure 3: Enhanced regions of GC×GC–TOF‐MS surface charts showing the identification of three features that can be used to discriminate between the expensive and inexpensive curry powders and the identification of these features using TOF mass spectra (red) compared with the NIST library (blue).
Conclusions
This study has shown that high‐capacity sorptive extraction in conjunction with GC×GC–TOF‐MS and powerful data mining software provides comprehensive aroma discovery. These probes enable robust and sensitive sampling and the flexibility to perform both headspace or immersive sampling; the enhanced separation of GC×GC compared to 1D GC provides a greater insight into sample composition; the TOF mass spectrometer enables confident identification of key quality markers; and the time‐saving untargeted workflows in the chemometrics platform automatically uncover the significant differences between complex aroma profiles.
References
1. I. Hazim, K. Younus, and F.T. Abachi, Iraqi J. Pharm. 16(1), 22–35 (2019).
2. J.‐Q. Cu, F. Perineau, and G. Goepfert, J. Essent. Oil Res. 2(2), 91–92 (1990).
3. K.M. Brodowska, A.J. Brodowska, K. Smigielski, and E. Lodyga‐Chruscinska, Eur. J. Biol. Res. 6(4), 310–316 (2016).
4. The Good Scents Company Information System, www.thegoodscentscompany.com, search facility accessed on Nov. 7, 2020.
Laura McGregor received an M.Chem. in chemistry from the University of St Andrews, UK, followed by an M.Sc. in forensic science at the University of Strathclyde, UK. Her Ph.D. in environmental forensics, also at the University of Strathclyde, focused on the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC−TOF-MS. In her current role at SepSolve Analytical, she specializes in the application of GC×GC and TOF-MS to challenging applications.
Stefan Koschinski received his degree in food chemistry from Goethe University Frankfurt, Germany. His Ph.D. at the Dienstleistungszentrum Ländlicher Raum Rheinpfalz and University of Kaiserslautern followed, which was focused on wine analysis, applying different sample preparation, enrichment, and separation techniques as well as chemometrics. He joined Markes International in 2012 and works as a senior product specialist with a regional focus on Germany, Austria and Switzerland.
Elinor Hughes obtained her B.Sc. in chemistry and Ph.D. in organic chemistry at Bangor University, UK. After working for a chemical manufacturing company for three years, she moved to the Royal Society of Chemistry where she worked in journals publishing for six years and on Chemistry World magazine for four years. This was followed by five years as a freelance copyeditor and science writer. Her current role is technical copywriter at Markes International.
E-mail: hello@sepsolve.com
Website: www.sepsolve.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.
Fundamentals of Benchtop GC–MS Data Analysis and Terminology
April 5th 2025In this installment, we will review the fundamental terminology and data analysis principles in benchtop GC–MS. We will compare the three modes of analysis—full scan, extracted ion chromatograms, and selected ion monitoring—and see how each is used for quantitative and quantitative analysis.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)