HPLC Systems and Components Introduced at Pittcon 2012: A Brief Review
LCGC North America
Highlights of some of the new HPLC and related technology showcased both before and at this year's conference
One of the world's premier conferences and expositions on laboratory science, the Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy (Pittcon) is a showcase for the latest advances in laboratory instrumentation and technology, attracting all of the major high performance liquid chromatography (HPLC) vendors in addition to more than 900 exhibiting companies from around the world. Pittcon is a great place for vendors to introduce, and scientists to see, touch, evaluate, compare, and hear all about the latest in HPLC technology. This installment will highlight some of the new HPLC and related technology showcased both before and at this year's conference.
This year, the Pittsburgh Conference on Analytical Chemistry & Applied Spectroscopy (Pittcon) was held on March 11–15 at the Orange County Convention Center in Orlando, Florida. The conference, which annually attracts more than 15,000 attendees from industry, academia, and government from 90 countries worldwide, is sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh. Pittcon 2012 had more than 900 exhibitors registered to appear in more than 1800 booths. Upwards of 2000 technical presentations and nearly 100 short courses also were given. The conference provides a great opportunity for vendors to expose new high performance liquid chromatography (HPLC) products to both new and existing customers, and with this fact in mind, many HPLC vendors have coordinated their new product development cycle around Pittcon for years, introducing new technology at the conference for the first time. Although the proliferation of smaller specialized conferences has in recent years altered this approach, for example, many new mass spectrometry (MS) product introductions are now made at the annual Conference on Mass Spectrometry and Allied Topics (ASMS), Pittcon still remains a must-go-to meeting to see the latest and greatest in HPLC and related technology. However, this year marked the first time that at least one HPLC vendor decided to go completely virtual, and instead of attending Pittcon, chose to offer a series of local seminars and on-line web-based e-conferences instead. For the attendees, the conference provides a venue to evaluate the latest instrumentation, compare vendors, participate in product demonstrations, and speak with technical staff to resolve problems or investigate potential applications. It will be interesting to see how things play out in the virtual world.
At this years' Pittcon, several vendors introduced new systems, software, and components, as well as product line extensions. In this installment, I'll review some of the new HPLC and related instrument technology shown virtually and physically at the conference. Table I lists the company introductions that are reviewed in this column. (New column and sample preparation technology will be reviewed by Ron Majors in the April and May installments of Column Watch.)

Table I: Summary of systems and components reviewed in this article
Supercritical Fluid Chromatography
Supercritical fluid chromatography (SFC) has been commercially available since about 1982, but more recently it has gained in popularity because of advances in technology and applications to several difficult separation challenges (for example, the separation of chiral compounds). SFC is a form of normal-phase chromatography that is used for the analysis and purification of low- to moderate-molecular-weight, thermally labile molecules. The theory and principles of SFC are similar to those of HPLC; however, SFC can be differentiated from other chromatographic techniques (gas chromatography [GC] and HPLC) by the use of a supercritical fluid as the mobile phase and, therefore, the entire chromatographic flow path must be pressurized. SFC has several advantages compared to GC and HPLC methods, including rapid separations without the use of organic solvents, reduced use of organic additive chemicals, and the use of carbon dioxide collected as a by-product of other chemical reactions (or collected directly from the atmosphere), thereby contributing no new chemicals to the environment and promoting "green" chemistry. In addition, SFC separations can be done faster than HPLC separations because the diffusion of solutes in supercritical fluids is about 10 times greater than that in liquids (and about three times less than in gases). The lower diffusion results in a decrease in resistance to mass transfer in the column and allows for fast, high-resolution separations. Compared to GC, capillary SFC can provide high-resolution chromatography at much lower temperatures. This allows for the fast analysis of thermolabile compounds. Many vendors have recognized these advantages and have started to offer SFC instruments on existing HPLC and ultrahigh-pressure liquid chromatography (UHPLC) platforms.
Agilent offers three points of entry into SFC: the original 1260 Infinity Analytical SFC instrument; a Hybrid SFC/Ultra High Pressure Liquid Chromatography (UHPLC) system; and an upgrade path based on the Aurora Fusion A5 Evolution system that converts existing Agilent 1100 and 1200 HPLC systems to SFC. The Agilent hybrid SFC/UHPLC system is based on the 1260 Infinity Analytical SFC system, but adds both a quaternary or binary HPLC pump and a two-position 10-port valve to be able to switch between UHPLC and SFC and provide true orthogonal separations at pressures as high as 9000 psi. The hybrid system is capable of screening methods by switching between SFC and UHPLC in a single run sequence, significantly reducing cost (only one system to purchase), laboratory footprint, equilibration time, and instrument-to-instrument variability. Figure 1 shows an example of both an UHPLC and a SFC separation of a mixture of polycyclic aromatic hydrocarbons (PAHs) on the hybrid system.
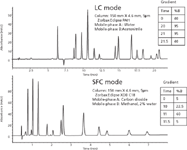
Figure 1
The new Aurora SFC Fusion A5 Evolution system is built on the original Aurora SFC Fusion A5 instrument and features a combination of optimized pumping technology with an ultralow-noise back-pressure regulator. The upgrade kit allows full conversion of an Agilent 1100 or 1200 series HPLC system to a full analytical SFC system that moves SFC smoothly into routine laboratory analyses with enhanced features for full qualification in a good laboratory practice (GLP) or good manufacturing practice (GMP) environment. With cost containment being the focus of many companies, conversion can reduce the overall cost of entry. Four orders of magnitude of dynamic range provide accurate quantitation of 0.01%-level impurities, high precision, HPLC-like sensitivity, and UHPLC speed using UHPLC columns. The system is fully compatible with the manufacturer's chromatography data system software.
At past shows, Waters has showcased its SFC system, the Waters Acquity UPSFC system that is built on the Waters UPLC sub-2-µm particle chemistry platform. The UltraPerformance SFC (UPSFC) system is designed to incorporate all of the advantages of SFC, in addition to superior separation, resolution, and speed afforded by the sub-2-µm particle chemistry platform. This year, Waters introduced the UltraPerformance Convergence Chromatography Acquity UPC2 system, which gives scientists the ability to precisely vary mobile-phase strength, pressure, and temperature. With the ability to fine-tune the resolving power and selectivity of the system, scientists can exercise better control over the retention of analytes for separating, detecting, and quantifying structural analogs, isomers, and enantiomeric and diasteriomeric mixtures — all compounds that are often difficult to separate by any other means. Its key features include
- A 10-µL fixed-loop injector for partial-loop injections of between 0.5 µL and 10 µL, which preserves sample and eliminates the need to change sample loops.
- Reduced system volume, which enables shorter run times, optimizes gradient performance, reduces band broadening, and enables the use of small-particle-size columns.
- Cosolvent and column switching for more flexible method development, and faster solvent and column screening.
- Gradient accuracy and precision for retention time reproducibility.
- Improved optical detection and MS compatibility for conducting both qualitative and quantitative analyses.
UHPLC
Waters showcased its second-generation UHPLC instrument called the Acquity UPLC H-Class system at Pittcon in 2010. Last year, Waters introduced three offerings in this area, the Acquity UPLC H-Class Bio System, the nanoAcquity UPLC System with 2D Technology, and the nanoAcquity UPLC system with HDX Technology. New for 2012 is another platform extension, the Acquity UPLC I-Class system, which is a binary system designed for MS. The I-Class system features reduced system volume and carryover. Reduced system volume significantly decreases dispersion rates for reproducibly higher resolution and peak capacity, and the resulting small peak volumes extend the sensitivity of any mass spectrometer. Additionally, low dispersion allows reduced separation cycle times without impacting resolution even for gradients lasting less than 1 min. Depending on the application, users can choose either a high-precision, low-dispersion fixed-loop sample manager (injector), or a low-dispersion, variable-volume sample manager with a flow-through needle design, which is designed to deliver high-precision injections, excellent sample recovery, and low sample carryover to optimize the performance for mass detectors. The I-Class system offers the capability to switch between two columns with independent thermal zones that can be operated from 4 °C to 90 °C. The column manager can be configured with interchangeable valves for serial column runs when configured with a second Acquity pump to support two-dimensional separations. Figure 2 illustrates an example of a separation from a USP monograph for the analysis of irbesartan that was scaled from HPLC to UHPLC, comparing the scaled separation on the I-Class system to a standard UHPLC system. While providing linearity over four orders of magnitude, an increased signal-to-noise ratio (S/N) is also observed for both the main component and a minor 0.05% related compound impurity.
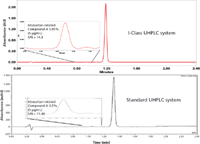
Figure 2
Micro HPLC
Eksigent, part of AB Sciex, introduced the new ekspert microLC 200 system, a dedicated splitless microflow UHPLC system. Optimized for integration with AB Sciex mass spectrometers with control by Analyst software (while still operating as an inlet for any vendors mass spectrometer), it offers increased sensitivity over analytical flow UHPLC, faster separations, and reduction in sample and mobile-phase consumption in a small footprint. The microLC binary gradient pumping system operates at 5–200 µL/min, at pressures up to 10,000 psi. Microfluidic Flow Control technology based on actual flow rate feedback to a rapidly adjustable pressure source reportedly delivers flow rate precision better than 5% RSD. Featured applications include peptide analysis from digested liver microsomes, and pharmaceutical and personal care products at trace levels in wastewater.
HPLC Detectors
Agilent introduced a new photodiode-array detection (DAD) module, in what the company has termed the 1200 Infinity Series High Dynamic Range (HDR) DAD system. The system takes advantage of Agilent's Optofluidic Waveguides Max-Light flow cells using total internal reflection in a noncoated fused-silica fiber detector cell. By combining the signals from two diode-array detectors with different pathlength Max-Light flow cells, the system allows detection and quantitation of all components in a sample in a single run — making it ideal for the analysis of mixtures with widely different concentration levels. Two model 1260 or 1290 Infinity diode-array detectors are deployed; one is fitted with a 3.7-mm pathlength flow cell, and the other has a longer 60-mm pathlength flow cell. The signal from the longer-pathlength cell is used to quantitate low concentrations, and the signal from the shorter pathlength cell is used for the high concentrations. Specially developed algorithms are designed to correct for the different retention times and compute these signals to expand the linear dynamic range by a factor of 30 and sensitivity by 8–10 times compared to earlier generation Agilent diode array detectors. An example of the use of the HDR DAD system is shown in Figure 3 for the analysis of a pharmaceutical formulation that has two active pharmaceutical ingredients (API). The concentrations of API 1 and API 2 are widely different and previously two separate runs with different injection volumes were necessary to quantitate both APIs and their respective impurities. With the HDR detection system both APIs and all impurities can be quantitated in one run, reducing sample turnaround time and boosting laboratory productivity.
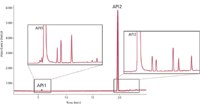
Figure 3
HPLC Autosamplers
Shimadzu introduced the SIL-30ACMP UHPLC autosampler, which combines with the optional CTO-30AS column oven as a front end for liquid chromatography–mass spectrometry (LC–MS) and LC–MS-MS systems. The autosampler holds up to six plates for increased capacity and up to 2304 samples when using 384-well plates. It has an open-access design that permits users to access the sample racks at any time, except during the injection process. Low carryover is maintained with a modified rinse mechanism, minimizing sample contamination. With an injection operating time of 7 s and a cycle time of 14 s, Shimadzu claims it is the "world's fastest autosampler." The optional CTO-30AS column oven mounts directly on the side for close proximity to the autosampler and can be positioned from the vertical to the horizontal to minimize tubing lengths, which is important for reducing extracolumn band broadening effects in UHPLC applications.
Software
Waters introduced NuGenesis 8 software featuring Laboratory Execution (LE) Technologies, a comprehensive data management and workflow system designed to support the entire product life cycle from discovery through manufacturing. The user-centric platform comprises NuGenesis SDMS, a compliant-ready data repository, and NuGenesis ELN, a flexible electronic laboratory notebook. The software's sample management feature allows analytical laboratories to manage and track samples and test requests through their entire life cycle, including transferring sample information from a laboratory information management system (LIMS) or systems applications and products (SAP) automatically into the electronic laboratory execution (LE) methods along with the specifications for the product to be tested. Data retention and legal hold capabilities allow organizations to manage the entire scientific data life cycle based on the organization's record retention policies from beginning to end in a compliant, secure environment. For example, if the record retention policy at an organization is to keep a certain type of record for five years, then purge records beyond five years, the system can automatically manage this process. However, if any records are undergoing legal consideration, then a legal hold flag is placed on these records and they are retained until legal consideration has concluded.
Electronic LE methods (predefined templates) that guide analysts through standard operating procedures (SOP) for pharmaceutical quality control (QC) testing also are a part of NuGenesis 8, providing direct interface to instruments and software platforms used for QC testing such as balances, pH meters, reagent databases, LIMS, enterprise resource planning (ERP) systems, and chromatography data systems. With instrument and consumable inventories you can ensure that analysts use only valid or calibrated instruments and consumables throughout a given analysis. Automated fit-for-use checks avoid the inadvertent use of expired chemicals or solutions as well as noncalibrated instruments and provide a single focal point for test result review and approval. NuGenesis 8 also manages data flow between laboratory instruments and information systems such as LIMS and ERP, increasing the efficiency of laboratory workflow and reducing waste and variability through information management standardization by
- providing quick and easy data accessibility for research and intellectual property management,
- facilitating product development and delivery by improving collaboration between functional groups and across multiple geographies, and
- serving as a long-term solution for data preservation and retention management.
Conclusion
The information in this review is partially based on manufacturers' responses to a preconference questionnaire mailed in late 2011. I used the information received in the questionnaires that were returned, as well as information from personal communication to try and make this review as comprehensive as possible. But keep in mind that because of the fact that some manufacturers did not respond to the questionnaire, or do not release preshow information, and the sheer size of the conference this report cannot be considered an exhaustive listing of all new products that were introduced in Orlando, Florida. I'm sure there were a lot of additional noteworthy items at Pittcon, but time, space, and the scope of this column prevents me from covering them all in detail. I'm bound to have missed a few items or details, and I apologize for any omissions. In 2013, Pittcon is scheduled to take place March 17–21 in Philadelphia, Pennsylvania. I hope to see you there!
Michael Swartz "Innovations in HPLC" Editor Michael E. Swartz, PhD, is Principal Scientist in Analytical Development at Ariad Pharmaceuticals, Cambridge, Massachusetts, and a member of LCGC's editorial advisory board. Direct correspondence about this column to "Innovations in HPLC," at lcgcedit@lcgcmag.com.

Michael Swartz

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









