HPLC-Based Measurements of Various Lipophilicity Parameters to Aid Drug Design
LCGC North America
Fast, reliable measurements of lipophilicity has a great impact on the compounds' selection process at an early stage of dug discovery. The guest columnit examines HPLC methods for this purpose.
Several models to predict developability include lipophilicity as a molecular property. Therefore, fast, reliable measurement of lipophilicity has a great impact on the compound selection process at an early stage of drug discovery. High performance liquid chromatography (HPLC) provides an automated platform to measure various types of lipophilicity based upon the compounds' retention time obtained on hydrocarbon-coated, protein-coated, or artificial membrane–coated stationary phases. The acid–base character of the compounds also can be revealed by performing the retention time measurements with mobile phases at acidic, neutral, and alkaline pHs. The HPLC-based biomimetic lipophilicity measures proved to be useful to develop models for adsorption, distribution, permeability, solubility, and toxicity.
To reduce attrition and cost of development of new drugs, various developability properties are now taken into account at a much earlier stage in the drug discovery process. Lipophilicity of the compounds plays an important role in various developability criteria, such as oral absorption, central nervous system penetration, and pharmacokinetic parameters. The lipophilicity of the drug molecules governs their partition to various lipids and protein phases and thus, reduces the free drug concentration at the active site. A proper balance between the specific binding potency and nonspecific partition of the compounds is of great importance in the design of developable effective drug molecules. Therefore, fast, reliable measurements of lipophilicity are gaining increasing importance in the early stage of drug discovery. Lipophilicity of a compound is characterized most often by its partition coefficient in octanol–water solvent system (logP). However, it has been recognized that one lipophilicity measure cannot explain a compound's distribution in various in vivo compartments (1,2). High performance liquid chromatography (HPLC) provides an easily automated, reliable, and accurate way to determine the concentration of a compound in various solvents used for the measurements of partition coefficients. However, the technique has even greater potential to determine the partition properties of compounds based upon their retention times. The chromatographic retention directly relates to the compound distribution between the mobile and the stationary phases. Various stationary phases can be used such as normal paraffin hydrocarbons, immobilized octanol, and biomimetic phases such as immobilized artificial membrane (IAM), human serum albumin (HSA), alpha-acid-glycoprotein (AGP),and so forth, and the pH and polarity of the mobile phase also can be altered. Thus, the technique is easily applicable to measuring other than octanol-water partition coefficients (3).
Methods
Generic gradient HPLC methods have been developed to measure lipophilicity of compounds using a reversed-phase, C18 stationary phase with acidic, neutral, and alkaline mobile phases (4), as well as using immobilized artificial membrane (IAM) phases (5) and chemically bonded protein phases (HSA and AGP) wit neutral (pH 7.4) mobile phases (6). By using acetonitrile gradients on the reversed-phase column and on the IAM stationary phase, compounds with a wide range of lipophilicity can be eluted within 5 min. An isopropanol gradient is applied on the protein phases to promote elution of even strongly bound compounds within 10 min. The chromatographic systems are calibrated by a set of molecules with known properties. The calibrated and, thus, converted retention times are used to derive the chromatographic hydrophobicity index (CHI) on the C18 and IAM stationary phases. The chromatographic retention obtained on the protein phases is converted to percent binding or the related binding constant (log K). A parallel four-way HPLC system has been set up to measure four partition properties of a compound in a single chromatographic run.
Results
Several hundreds of known drug molecules with known lipophilicity, plasma protein binding, passive permeability, and in vivo human volume of distribution have been characterized by the HPLC methods to validate its use for early candidate profiling. The CHI values obtained at pH 7.4 show good correlation with measured octanol–water distribution coefficients (mlogD) for various project compounds, as shown in Figure 1.
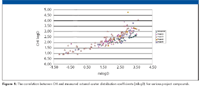
Figure 1
The CHI values obtained at three pHs (acidic, neutral, and alkaline) can reveal the acid–base character of the compound shown in Figure 2. Compounds are more lipophillic at the pH when they are partitioning in unionized form. The difference of CHI values at different pHs can be used to identify the presence of positive and negative charges at physiological pH.
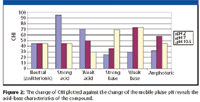
Figure 2
The plasma protein binding measured by ultrafiltration or equilibrium dialysis showed excellent correlation with the HSA) binding and AGP binding obtained from the gradient retention times by the HPLC method (Figure 3.)
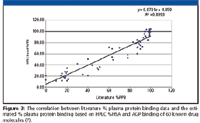
Figure 3
The method is capable for reliable ranking of molecules at the high binding region (above 95% bound), therefore, it can be applied to construct a structure–binding relationship and identify part of the molecules that can be modified to reduce binding without affecting the potency. For example, introducing and properly positioning a polar moiety into a nonpolar substituent generally reduced the average binding of analogs in a project series by 6% (see Figure 4.)

Figure 4
The compounds' retention on an IAM stationary phase reveals the compounds' partition properties with phospholipids, which have been shown to model membrane permeability and blood–brain barrier distribution (7,8). Although this process also is governed by lipophilicity, significant differences have been revealed between protein binding and IAM interaction as shown in Figure 5. It has been found that, in general, positively charged compounds bind more strongly to an IAM phase and negatively charged compounds bind more strongly to HSA. Also, compounds with positive charge and strong membrane binding have larger volumes of distribution than negatively charged ones, thus, in vivo volume of distribution can be modeled by these two experimental data (10) (Figure 6). Table I shows the HPLC-based properties and in vivo properties of a few selected drug molecules as an example. Based upon structure–binding relationships, the property of molecules can be altered to have desirable distribution and concentration at the pharmacologically active site.
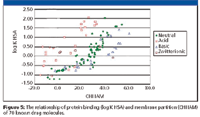
Figure 5
Conclusion
HPLC-based measurement of various properties of active molecules in the secondary screening provide valuable help in the drug design process. From the various measured data, the lipophilicity of the compound at physiological pH, the lipophilicity of the uncharged species, the presence of charge at physiological pH, the fraction bound in plasma, the fraction bound in phospholipids, and the volume of distribution can be revealed. As the property data are based upon retention time measurement, they are highly accurate and do not depend upon concentration. Impurities do not alter the results as they are separated from the main component during the HPLC process.
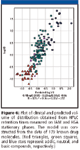
Figure 6
The lipophilicity of closely related analogs (diastereomers, enantiomers, or isomers) can be distinguished easily. Using parallel HPLC systems, these measurements have been carried out in a high-throughput, automated procedure. More than 10,000 compounds at the lead optimization stage were characterized in a year and provided valuable developability information for candidate selection.
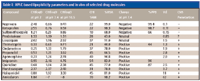
Table I: HPLC based lipophilicity parameters and in vivo of selected drug molecules
Klara Valko is Investigator in Analytical Chemistry, Physicochemical Characterization at the GlaxoSmithKline Medicines Research Centre in Stevenage, Herts, UK, and Honorary Professor at the London School of Pharmacy.
References
(1) J. Hodgson, Nature Biotech. 19, 722–726 (2001).
(2) H.Van de Waterbeemd, D.A. Smith, K. Beaumont, and D.K. Walker, J. Med. Chem. 44(9), 1313–1333 (2001).
(3) D.E. Leahy, J.J. Morris, P.J. Taylor, and A.R. Wait, Model Solvent Systems for QSAR. 4. The Hydrogen Bond Acceptor Behaviour of Heterocycles. 7, 743–750 (1994).
(4) K.J. Valko, J. Chromatogr. 1037, 299–310 (2004).
(5) K. Valko, C.M. Du, C. Bevan, D. Reynolds, and M.H. Abraham, Curr. Med. Chem. 8, 1137–1146 (2001).
(6) K. Valko, C.M. Du, C. Bevan, D. Reynolds, and M.H. Abraham, J. Pharm. Sci. 89(8), 1085–1096 (2000).
(7) K. Valko, S. Nunhuck, C. Bevan, M.H. Abraham, and D.P. Reynolds, J. Pharm. Sci. 92, 2236-2248 (2003).
(8) B.H. Stewart and O.H. Chan, J. Pharm. Sci. 87, 1471–1478 (1998).
(9) A. Taillardat-Bertschinger, P.-A. Carrupt, F. Barbato, and B. Testa, J. Med. Chem. 46(5), 655–665 (2003).
(10) F. Hollosy, K. Valko, A. Hersey, S. Nunhuck, K. Gy, and C. Bevan, J. Med. Chem. 49, 6958–6971 (2006).
Tim Wehr

Tim Wehr
"Directions in Discovery" editor Tim Wehr is staff scientist at Bio-Rad Laboratories, Hercules, California. Direct correspondence about this column to "Directions in Discovery," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.













