How to Optimize Method Repeatability in the Analysis of Small Molecules by Capillary Electrophoresis
Why is method repeatability so important in capillary electrophoresis?
The transfer of capillary electrophoresis (CE) methods from the research and development (R&D) laboratories to routine quality control (QC) laboratories requires that the methods can be repeated in laboratories outside the method development/validation laboratories and possibly on a variety of CE instruments. Method details are also included in marketing applications to regulatory authorities who may select to run the methods.
CE historically had a reputation for lack of reproducibility and precision. CE methods were initially developed and reported but attempts to repeat them from literature were often unsuccessful. This perceived lack of repeatability hampered the implementation of routine CE methods because they were considered a high risk.
The early CE methods developed and validated by industrial laboratories in a variety of sectors — including forensic, clinical and pharmaceutical — were found to be more reproducible because these methods were generated to produce important data that would stand considerable scrutiny.
The lessons learnt from the early work in industrial laboratories have been incorporated into current methods and CE repeatability has improved considerably. The performance of CE instruments has also improved. CE methods have now been incorporated into numerous regulatory filings for pharmaceuticals and successfully implemented into routine QC testing and batch release. CE is also used routinely in forensics and clinical laboratories. This article will discuss the optimization of repeatability using CE for small molecule analysis. The next installment of "CE Currents" will discuss repeatability for large molecule analysis by CE.
Are there Reported Examples of Successful Inter-laboratory Repeatability Exercises in Small Molecule CE Analysis?
Many CE methods have been successfully transferred into QC laboratories. However, the confidential nature of work within industrial laboratories means this repeatability data is not published. However, some inter-laboratory repeatability exercises have been reported (1–7).
A group of eight UK-based pharmaceutical companies conducted a three-part interlaboratory repeatability exercise. This was coordinated through the UK Pharmaceutical Analysis Science Group (http://www.pasg.org.uk/).
The initial method (1) involved the chiral separation of clenbuterol using a low pH phosphate buffer containing cyclodextrin. All companies were able to obtain the separation on the first injection using their own equipment and reagents. One of the participants had never even used the equipment before! Precision and linearity was also successfully assessed and reported. The second method (2) involved use of a micellar method (a simple borate buffer containing 50 mM SDS) to test the dose uniformity of paracetamol content in capsules. All companies obtained the separation and obtained good precision and agreement of results across the group (304 mg/capsule) and with the label claim (300 mg/capsule) and HPLC results (304 mg/capsule) for the batch — an internal standard (IS) was used in the CE analysis.
The final exercise (3) involved quantification of the sodium ion content in the sodium salt of a cephalosporin drug substance. Quantification used potassium as the IS and a simple low pH buffer containing imidazole to provide the background signal to perform indirect UV detection at 214 nm. All the companies again reported good accuracy (CE assay result was 5.46%w/w versus theoretical content of 5.47%w/w) and precision results. High sample concentrations were used to give large peaks which helped with quantification. These three methods were also successfully used for the practical training of FDA scientists.
Workers at the University of Jena and Astra Zeneca used statistical experimental designs to optimize and validate a chiral CE method (4) for the impurity profiling of calcium levofolinate including the enantiomeric impurity. The method allowed detection of all the required impurities at 0.1%. The method was very highly described; in particular the preparation of the electrolyte. Various electrolyte and method combinations were assessed to understand the method robustness. Robustness testing was across instrument type and laboratories. The method was fully validated to International Conference on Harmonization (ICH) standards.
Good repeatability of a micellar CE method (5) used to determine seven antibiotics in pharmaceuticals and feedstuffs. The researchers conducted a very thorough evaluation of factors affecting quantification, for example, noticing that peak height variation was higher in summer when evaporation losses from the samples in the non-temperature controlled autosampler increased.
Eleven participating academic laboratories attempted to repeat a cyclodextrin-based non-aqueous CE method (6) for quantifying R-timolol content in S-timolol maleate. The trail was designed following ISO guideline 5725-2. Not all participants were able to reproduce the method performance. This was because of differences between the separations obtained on different types of CE instruments — this is discussed in more detail in a later section. Relative migration time precision was measured (n = 6 injections) and results ranged from 0.1–0.7% relative standard deviation (RSD).
A precision study on CE method for metacycline (7) was performed in an inter-laboratory study across ten academic laboratories. The method conditions were complicated and involved on-capillary chelation with ethylene diamine tetra acetic acid (EDTA), an exact pH of 10.35 and a buffer containing 13%v/v of methanol. Only seven of the laboratories produced sufficient data to be included in the data analysis. A low level of isochlortetracycline was used as an IS that generated precision issues. Currents varied across the various instruments (80–150 µA), which had profound effects on the migration times and selectivity achieved — this was a result of the increased temperatures within the capillaries at higher operating currents. The temperature affects both the flow-rate and the level of complexation/chelation. The method was then redeveloped to improve performance. Modifications included use of a higher IS concentration and optimization of the electrolyte — the method showed improvements but could still only be operated on two of the three instruments.
What can be Done to Improve Method Repeatability?
The flowchart in Figure 1 shows a method lifecycle to ensure good method repeatability. This is common to both CE and HPLC methods.

Figure 1: Method lifecycle to ensure good method repeatability.
Design Method: The design method is the initial step where the purpose of the method is agreed and method development begins. Method development should ideally be performed on an actual sample because sample constituents may affect the separation. For example, the ionic strength of a sample solution may be high which will alter peak shape; this would not be observed for a standard simply dissolved in water. Often generic conditions (such as 50 mM phosphate pH 2.5, 15 mM borate or a standard micellar or microemulsion buffer) provide the required resolution. The method should be kept as simple as possible to maximize repeatability. For example, a low pH separation of two basic compounds relies solely on differences in their charge/size ratios which are physiochemical properties and are very robust. Similarly a simple borate (pH 9.3) buffer containing sodium dodecyl sulphate (SDS) micelles separates neutral compounds based on their solubility which, again, is very robust. The addition of solvents, for example, complicates methods as evaporation losses can cause variability. If a solvent must be added, then a less volatile solvent such as DMSO will give less variance. Variance in the purity of additives. such as cyclodextrins (8), can also affect method repeatability — therefore assess unmodified native cyclodextrins initially before moving to derivatized cyclodextrins that have variable degrees of substitution and each lot will give a slightly different separation.
Use of kits or dynamic column coating approaches also greatly improves method repeatability and should be considered if appropriate to the separation.
Optimize Precision: The use of an IS greatly improves method performance and enhances repeatability (9). The selection of an IS is generally not complicated, especially if generic method conditions are used. Precision is also related to peak size and greater precision will be obtained when the analyte (and IS) peaks are large. Sample preparation robustness is again improved using an IS and avoiding small transfer/sample volumes.
Assess Robustness: This is a key activity to ensuring method repeatability and should ideally be performed during method development using an actual sample. Use of experimental designs during method development allows mapping of the space around the method conditions (4). For example if a method is set to run at pH 3.5 with a 50 mM buffer, then it is useful to know what performance will be achieved when the buffer is, for example, 55 mM/pH 3.3, 45 mM/pH 3.7 or 50 mM/pH 3.7. Other factors to include in the design are voltage, temperature and sample concentration.
Data is generated by running attended sequences of methods on the instrument that equate to the various conditions being assessed. The results are then usually presented graphically as contour maps (4). A detailed robustness evaluation allows method limits to be included in the method, for example, the method may state pH 3.5 (± 0.2), 50 mM (± 5 mM) phosphate buffer. The results from the robustness study also show how the method fails and helps guide the selection of system suitability parameters.
If a method will be run on more than one CE instrument type then this should also be included as a factor in the robustness study because this can have a considerable impact on method performance — see later section.
Document Method: This is another key activity to ensuring method repeatability. It is vital to document all aspects of the method (4) so that it can be successfully repeated. It is insufficient to state use of a pH 2.5, 50 mM phosphate buffer because it does not specify how the buffer is prepared — it is necessary to state (10) what the initial buffer solution is (For example, 50 mM sodium dihydrogen orthophosphate), and what solution is used to adjust the pH. Failure to fully document buffer preparation leads to buffers of variable ionic strength that produce variable levels of current and flow inside the capillary that will spoil the repeatability. The filtration procedure for the electrolyte should also fully defined (10) as this can impact the resulting buffer (For example, some of the SDS in micellar solutions can be adsorbed onto filters, resulting in lower concentrations of micelles and altered selectivity). Factors affecting sensitivity, such as the capillary slit width, use of bubble cells and even detector sampling rates, should all be included in the method. The exact dissolving solvent for sample and standards should also be specified as this can have pronounced effects on the separation performance — once the solvent is determined it should not be altered.
Transfer Method: This is another important step and is generally controlled via a transfer protocol which states pre-agreed testing and acceptance criteria for the results that will be generated. Method training is sometimes performed for complicated methods where an analyst from the transferring laboratory visits the receiving laboratory and assists the laboratory in using the method. The results for a series of testing by both the transferring laboratory and the receiving laboratory are statistically compared against pre-defined criteria.
Assess Method: This activity should happen periodically where an assessment of the method in routine use occurs. If there have been issues with the method then this should be assessed by suitable redevelopment, redefinition of system suitability parameters or tighter controls on the method.
Why is There a Need to Perform Inter-instrument Type Repeatability in CE?
There are technical differences between CE instruments which can have a significant effect on method performance. The technical differences between HPLC instruments are very small and as such have a very limited impact on the performance of methods. Therefore HPLC methods give similar performance when run on different HPLC instrument types. Table 1 details some of the issues that can arise when methods are transferred between different instrument types.
It is therefore important that method conditions are determined and fully documented for any instruments that the method will be used on. Regulatory submissions and methods often include two (or more) sets of operating conditions to allow use of multiple instrument types. As previously mentioned instrument type should be included in robustness studies if it is known that the method will be routinely run on more than one instrument type.
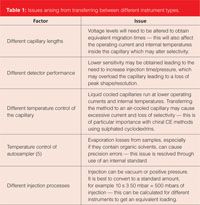
Table 1: Issues arising from transferring between different instrument types.
Conclusions
Method repeatability was identified as an issue in the early days of CE implementation and this somewhat hampered the introduction of CE because it was a perceived risk with the technique. Improved knowledge and experience, and improved instrumentation have improved the performance of CE methods and has lead to increased useage.
CE methods are sensitive to a variety of factors and these should ideally be assessed fully during method development by using experimental designs. If the method will be run on more than one CE instrument type then this should also be assessed during robustness studies.
Documentation of the final method parameters is important to ensure that the method can be fully replicated and should include important factors such as the pH adjustment procedure which may previously have been overlooked but have a pronounced impact on method performance. Use of validated kits and dynamic coating systems can also markedly improved method repeatability.
Many CE methods are now in routine use across a number of regulated industries following successful method transfer exercises. These transfers are confidential and therefore not published. It may seem that there is no evidence of CE method repeatability, but good method repeatability is possible and is being routinely achieved when good practices are followed.
Kevin Altria is an associate director in the pharmaceutical development department at GlaxoSmithKline. He is editor of "CE Currents " and a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to "CE Currents",LCGC Europe , Advanstar Communications, Bridgegate Pavilion 4A, Chester Business Park, Wrexham Road, Chester CH4 9QH, UK, or e-mail the the editor of LCGC Europe, Alasdair Matheson, at amatheson@advanstar.com
References
1. K. Altria et al., J.Chromatogr., 641, 147–153 (1993).
2. K. Altria et al., Chromatographia, 39, 180–184 (1994).
3. K. Altria et al., Chromatographia, 40, 47–50 (1995).
4. F. Sü et al., Electrophoresis, 25, 766–777 (2004).
5. R. Injac et al., Anal. Chim. Acta, 594, 119–127 (2007).
6. R.D. Marini et al., Electrophoresis, 27, 2386–2399 (2006).
7. T.D. Thi et al., Electrophoresis, 27, 2317–2329 (2006).
8. K. Altria, LGGC Europe, 23(1), 34–43 (2010).
9. K. Altria, LCGC Europe, 15(8), 588–594 (2002).
10. K. Altria, LCGC Europe, 20(4), 194–200 (2007).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.











