Is Headspace Sampling Quantitative?
Whether or not headspace sampling is quantitative depends on the information gleaned from the analysis
Confusion exists on the quantitative nature of headspace sampling because it is an equilibrium-based technique when done in the static mode, but not necessarily in the dynamic mode. To aggravate matters further, the concentrations of headspace compounds in common applications, like foods, flavors, or petroleum distillates, can easily vary by an order of magnitude or more. Thus, what defines quantitative may depend largely on the goals of the analysis. This month, we'll take a look at headspace sampling and its quantitative nature.
I've always commiserated to some extent with those working in the food and flavor industries. With almost any other consumer product, one can demonstrate superior performance in terms of speed, economy, cleanliness, pain relief, and so forth. But, with foods, if someone doesn't like a particular flavor, there's not much that can be done to win that customer. If one dislikes lemon flavor, they won't purchase lemon-scented products. We've all been involved in the great cilantro debate, right? Flavor and aroma are intertwined, and provide a challenging analytical problem. Aromas are typically considered devoid of matrix interactions, yet flavors are often intimately associated with the product and may be released over time, such as when chewing. The concentrations of compounds in the aroma or flavor can vary widely, as can other key properties, such as polarity and volatility.
I bring this up because headspace sampling is a staple in the analysis of foods, flavors, fragrances, petroleum distillates, and other applications involving volatile analytes. This brings to mind a conversation I had this past summer where the question arose, "Is headspace sampling quantitative?" My partner in conversation referred me to a recent interview with a renowned food chemist (1). In this interview, Prof. Gary Reineccius from the University of Minnesota rightly presented an argument on the need for analyte concentration to increase sensitivity, the limitations of commonly used headspace extraction methods, and the need for researcher to critically evaluate the literature as they plan their research. Thus, getting back to the question of "Is headspace sampling quantitative?" is somewhat akin to President Clinton's "It depends on what the meaning of 'is' is." To truly address the quantitative nature of headspace sampling, one must have more than a cursory understanding of the mechanisms of the nature of the headspace phenomenon, as well as an understanding of their individual analytical problem.
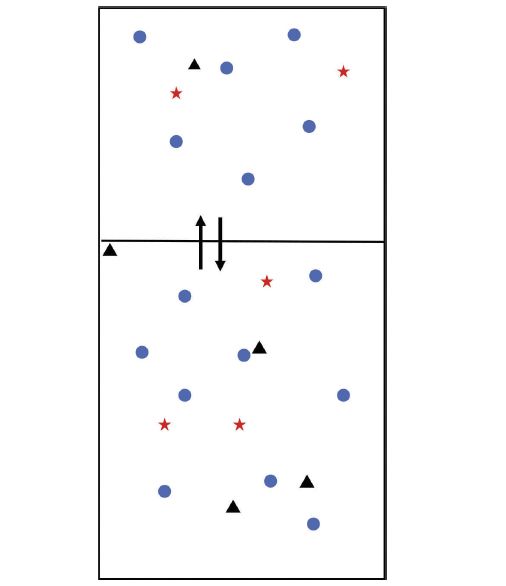
Figure 1: Depiction of a static (equilibrium) headspace system in which the blue circles (â) and red stars (â
) have equal volatility and the black triangles (â²) are less volatile. With each system component, the rate of evaporation equals the rate of condensation.
Basics of Headspace Sampling
Headspace refers to the vapor phase, typically, above a liquid or solid sample. Sampling this gaseous, or headspace, phase for injection into gas chromatography (GC) has several advantages. Since no organic solvent is used, the introduction of impurities to the analysis is minimal, there is no solvent peak which may obscure (due to coelution) analytes, the sample is not diluted, and the cost, waste, and environmental and health effects from solvent use are eliminated. As a basis for this discussion, we will assume that the system is at equilibrium- that is, the rate of evaporation of every component in the system equals the rate of condensation of all of the system components. This basic headspace equilibrium is depicted in Figure 1. For each component, we can define an equilibrium, or partition, coefficient:
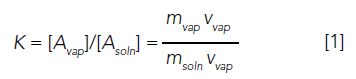
which relates the concentration in each of the two phases. In our example, note that the blue circles and red stars both have the same value of K. Even though their concentrations in each phase is different, the ratios of these concentrations are equal. On the other hand, the black diamonds have a much lower value of K. Since we are dealing with the liquid (solution) to gas equilibrium, we can equate this to the volatility of each component. So, the black triangles, with the lower K, are considered less volatile than the blue circles and red stars, which have equal volatility.
With this equilibrium system, samples may be removed for GC analysis without disturbing the system in any way. This is an example of static, or equilibrium, headspace sampling. In the simplest form of headspace sampling, a gas-tight syringe is used to remove a portion of the vapor phase, which is then injected into GC. As noted, one particular disadvantage, despite the simplicity, of this approach is the extremely large concentration range of analytes in the system, especially for flavors and fragrances. For example, Reineccius notes that coffee may contain over 900 volatile components, but simple headspace-GC may reveal only about 30 of these (1). Nevertheless, static headspace sampling can be considered a worthy measurement of aromas, as this is the vapor-phase component of a chemical system.
When coupled with GC characterization, headspace sampling is very quantitative in terms of accuracy, precision, and other quantitative metrics. What is missing are considerations related to a) sample concentration to increase analytical sensitivity, and b) performing a more comprehensive compositional analysis.
Increasing Analytical Sensitivity of Headspace Sampling
To increase sensitivity of analysis, we need to collect more of the analytes. One of the most common means, among the most common methods currently, is to add a third, sorbent phase to the system. Historically, this was performed by the use of bulk sorbents, such as Tenex or molecular sieves. This is currently done using solid-phase microextraction (SPME) and various derivatives, such as thin-film SPME and related devices. We will offer a brief description of the SPME approaches as an example of all approaches for achieving a greater sample amount collected. Each of these SPME-type devices introduces a third phase, and an additional equilibrium step, to the system. As equilibrium is reached, the analytes will partition to the sorbent, where they are collected, taken to the gas chromatograph, and thermally desorbed into the chromatographic inlet. With the newer types of SPME and related devices, increased sorbent mass or surface area allows for the collection of increasing amounts of analyte, improving the sensitivity. For example, Figure 2 demonstrates that higher recoveries are obtained at smaller partition coefficients compared with conventional SPME (2) as the amount of sorptive phase increases. For example, at a constant extracted amount, target analytes with lower partition coefficients can be isolated. For a compound of a given partition coefficient, dramatic improvements in amount extraction can be observed as the extracting (sorbent) phase amount increases. Take, for example, acenaphthylene with a log K of 3.17; only about 5% is expected to be extracted from aqueous solution with conventional SPME, while about 45% is predicted to be extracted with SPME Arrow (which contains 17 times the sorbent amount), and nearly 80% extraction is predicted using stir-bar sorbent extraction (SBSE). While the example presented in Figure 2 is for extraction of an aqueous solution, and SBSE is not generally used for headspace extractions, the principle of analyte concentration using greater sorbent amounts still holds. This method of using sorbent phases like SPME to collect headspace samples makes two key assumptions. First, it is assumed that the partitioning between the analyte in the vapor phase and the sorbent (extracting) phase is equal to the partitioning between the sample (solution) phase and the headspace vapor. Second, one must perform the extraction under conditions where each of the equilibria are achieved. This approach is becoming increasingly commonplace. Newer methods, including the SPME Arrow (CTC Analytics) and Sorbent Pen (Entech Instruments), are being developed to increase the sorbent capacity by increasing the sorbent mass or surface area, while maintaining a configuration easily amenable to GC injection, including via automation. It should be noted, however, that these means for increasing analyte sensitivity do not change the fundamental liquid-vapor equilibrium, so they may be considered applicable for aroma analysis. They are also quantitative from the accuracy and precision point of view; they merely collect and concentrate more sample for analysis. If we reconsider the case of the coffee aroma consisting of 900 components, with 30 compounds determined by standard headspace sampling, we can now see an increased number of less concentrated compounds, perhaps as many as 70-100 additional. The presence of these previously unseen compounds dilutes the presence of the major compounds, but since these newly observed compounds are in minor amounts, by what extent is the concentration of the major components diluted? We may get a different quantitative answer than in our conventional headspace sampling, but both are quantitative, and the more concentrated sample is more representative of the aroma being studied.
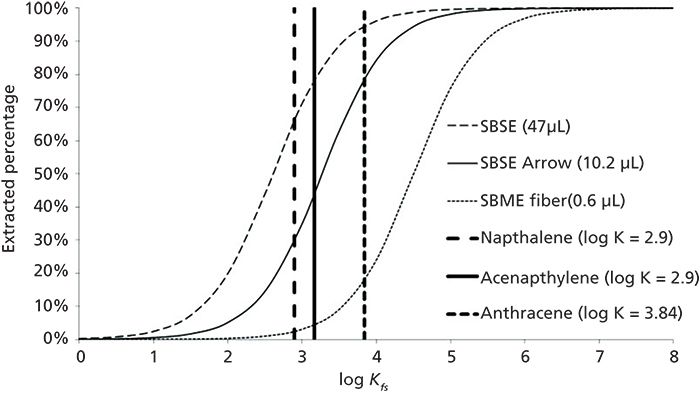
Figure 2: Theoretical extraction amounts using stir-bar sorptive extraction (SBSE), solid-phase microextraction (SPME) Arrow, and conventional SPME for selected polycyclic aromatic hydrocarbons (PAHs) calculated for a 19-mL aqueous sample using literature-based partition coefficients. Reproduced from reference (2).
Comprehensive Analysis
A more complete analysis, capable of providing a more comprehensive understanding of the sample composition, but altering the original (room temperature) aroma composition, involves disturbing the liquid-vapor equilibria to force more analyte into the vapor phase. This disruption may occur via the salting out effect, heating, flow of an inert gas, or application of a vacuum. We'll briefly look at each of these.
Salting Out
The well-known salting out procedure from liquid-liquid extraction and protein precipitation drives analytes between two phases, and can be used to move solution-phase analytes into the headspace by altering the ionic strength and solvation energy of the original (aqueous) solution. Ammonium and alkali metal salts, especially of hydrogen phosphates, nitrates, sulfates, acetates, and chlorides, decreases hydrophobic activity and solubility of nonpolar molecules in polar solvents, especially water, according to the established Hofmeister lyotropic series.
Heating
It makes sense that heating will increase the rate of evaporation, enhancing the mass transfer, and driving analytes into the vapor phase, thus increasing their concentration for subsequent collection. The problem lies in the fact that different molecules possess different volatilities and, thus, differing amounts extracted, as illustrated in Figure 3 (3). In this example, aromatic hydrocarbons are sampled from the headspace above olive oil samples using SPME. It can easily be seen that, as the sample is heated, the behavior of different sets of compounds changes, and not necessarily in an easily predictable manner. Despite this, headspace analysis is frequently performed at elevated temperatures, so the extraction temperature must be well-documented.
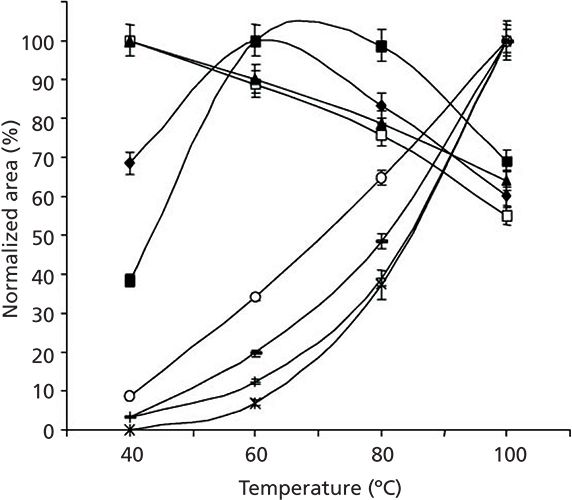
Figure 3: Thirty-minute solid-phase microextraction (SPME) extractions of aromatic hydrocarbon standards in olive oil as a function of temperature. Specific standards include (â¡) C1-benzene, (solid triangle) C2-benzene, (â) C3-benzene, (solid square) C4-benzene, (open circle) naphthalene, (–) acenapthene and acenaphthylene, (+) phenanthrene, anthracene, and fluorine, and (×) fluoranthene and pyrene. Reproduced from reference (3).
Inert Gas Flow
Headspace extractions are frequently made more exhaustive by applying a gentle flow of inert gas, such as helium or nitrogen. In a dynamic extraction, this flow passes over the surface of the (usually liquid) sample. The analytes that are volatilized and isolated in this manner must subsequently be trapped, usually cryogenically. This trapping may occur on a sorbent material, such as specially constructed trapping tubes or any of the various sorbent-based extraction techniques, or cryogenically trapped directly onto the head of the GC column. Purge and trap, or gas stripping, analysis is somewhat similar to dynamic headspace sampling, except that the flow of inert gas enters below the sample-vapor interface and is allowed to flow through the sample. Of course, with the flow of inert gas, whether in the dynamic or gas-stripping modes, the rate of evaporation will vary with each individual analyte type as observed with the other nonequilibrium techniques. With the addition of the gas flow, the most difficult additional step from a practical perspective is the analyte trapping.
Vacuum
Applying a vacuum to the headspace and re-establishing the equilibrium is an approach to headspace sampling that is only recently finding more broad use. By adding this additional experimental parameter, sampling times are shortened and more mild temperatures can be used. Recalling that Henry's law relates the partial pressure of a gas-phase compound with its solubility in a liquid, Psillakis (4) reports that, for compounds with low Henry's law constants, greatly improved extraction rates are observed with application of vacuum conditions compared with headspace sampling at or near atmospheric pressures. The recent tutorial by Psillakis (4) is very worthwhile reading for researchers interested in exploring this subject.
Is Nonequilibrium Headspace Sampling Qualitative?
In this section, we took a brief look at headspace sampling using applied conditions which altered the equilibrium system. This application of salts, temperature, inert gas flow, or reduced pressure rendered the subsequent extraction more exhaustive compared with the equilibrium headspace extraction, which were considered more representative of a sample aroma. From the perspective of quantitative analysis metrics, each of the methods discussed can be reproducible. They can be accurate, if the analyst understands the system being studied; that is, are the major components of an aroma of interest or the more minor compounds which can impart important sensory or chemical properties, or is an exhaustive, compositional analysis of interest? This is where the question of whether headspace sampling is quantitative becomes more dubious. The answer is, "Yes, but," as the analyst must have a thorough understanding of the system they are studying, and the implications of the headspace sampling conditions used.
Conclusion
Whether headspace sampling is quantitative depends on the information gleaned from the analysis. Rational arguments can be made stating that headspace is or is not quantitative. Thus, a simple answer does not exist, and one must have a deeper understanding of their analytical goals. In uncertain times like this, I like to go back to the fundamentals, which are, in this case, in an article published by Jennings and Filsoof (5) the same year I graduated from high school. In this study, a standard mixture was made with a number of solution components spanning a broad range of organic functional groups and polarities. Static headspace sampling, headspace sampling following salting out, trapping onto either Porapak Q or Tenax GC followed by a solvent wash, and simultaneous distillation-extraction were each compared with injection of the neat standard solution directly into GC. In this case, simultaneous distillation-extraction compared most favorably to direct GC injection of the standard solution. But this result is not as important as the ultimate conclusion, which I think still holds today relative to the question of whether headspace sampling is quantitative. Jennings and Filsoof state, "The results indicate that no single sampling system can be regarded as uniformly satisfactory, but that, depending on the sample and what the investigator wishes to study, one or another system may be superior."
References
(1) R, Muenz, Lab Manager 13(3), 44–47 (2018).
(2) A. Kremser, M.A. Jochmann, and T.C. Schmidt, Anal. Bioanal. Chem. 408, 943–952 (2016).
(3) S. Vichi, L. Pizzale, L.S. Conte, S. Buxaderas, and E. Lopez-Tamames, J. Chromatogr. A 1090, 146–154 (2005).
(4) E. Psillakis, Anal. Chim. Acta 986, 12–24 (2017).
(5) W.G. Jennings and M. Filsoof, J. Agric. Food Chem. 26, 440–446 (1977).
ABOUT THE COLUMN EDITOR
Douglas E. Raynie

Douglas E. Raynie "Sample Prep Perspectives" editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@ubm.com
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)