Green Chemistry: What Is It (and What Is It Not)? And How Does It Apply to Gas Chromatography (GC)?
Exploring the impact of green chemistry on analytical chemistry and GC is the focus of this instalment of "GC Connections".
Since the publication of the first tenets of green chemistry about 25 years ago, the conversation about sustainability and chemistry has slowly grown, and today seems at a fever pitch, as companies are adding sustainability officers, universities are adding curriculum, and chemistry-related processes being updated regularly. In this instalment, we will being exploring the impact of green chemistry on analytical chemistry and gas chromatography (GC). We will define green chemistry, and discuss what it is and what it is not. For example, it is not “chemistry lite”. We will then briefly review relevant literature on green analytical chemistry, and begin a conversation on green gas chromatography and the many areas in which GC-based methods can be made “greener”.
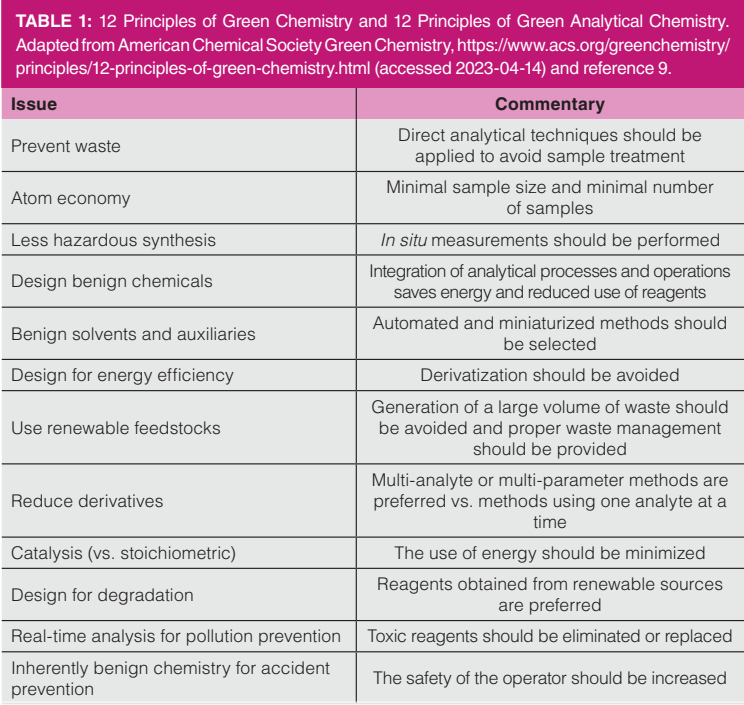
The 12 Principles of Green Chemistry were introduced in the late 1990s and have seen rapidly increasing interest over the past decade (1–3). Table 1 lists these principles of green chemistry along with principles of green analytical chemistry. More recently, the United Nations has promulgated their 17 Sustainable Development Goals (UN SDGs), which are guiding policymaking in governmental and corporate venues (4). The 12 Principles of Green Chemistry and the UN SDGs guide sustainable practices in the chemical sciences and in analytical chemistry research and development. Thinking about green chemistry and sustainability in chemical science can trace their roots to the early work of Lovelock, the inventor of the electron capture detector, and the classic book that is credited with starting the environmental movement, Silent Spring (5–7).
Not surprisingly, there has been much politics and public discourse around the broad topics of sustainability and the UN SDGs. The American Chemical Society (ACS) clearly states that green chemistry is “not politics”, “not a public relations ploy”, and “not a pipe dream” (3). For educators like myself, it is also not “chemistry lite”. Using the 12 Principles of Green Chemistry in teaching chemistry encourages the use of more modern techniques and ideas at earlier points in the curriculum, and green chemistry is clearly the future of chemical education.
Laboratory glassware over white | Image Credit: © zakrevski - stock.adobe.com

The 12 Principles of Green Chemistry have been adapted to analytical chemistry, with a useful recent review provided by Nowak, Wietecha-Pozluszny, and Pawliszyn (8). In 2013, Galuszka and co-authors outlined 12 Principles of Green Analytical Chemistry (also listed in Table 1) (9). Taken together, the principles of green and green analytical chemistry provide an interesting roadmap for analytical method development and optimization. While the 12 Principles of Green Chemistry are clearly geared towards synthesis and manufacturing, some clear parallels are seen in waste reduction, atom economy, reduced energy usage, miniaturization, and improved safety. Interestingly, and specific to gas chromatography (GC), both sets of principles call for elimination of derivatization. The analytical principles also call for multi-analyte methods, another principle that directly encourages chromatography.
Systems Thinking
Systems thinking, in which a process is considered holistically and can be broken down into individual steps, which are then evaluated for their overall impact on the process, related external processes, and sustainability, is another aspect of green chemistry and sustainability. The classic work by Meadows describes systems thinking in detail, with examples where the whole process can be negatively impacted by a seemingly positive change in one area (10). Analytical chemists already apply a form of systems thinking when developing methods, especially those with goals such as high speed, low cost, and easy to use. In developing a full method, we are taught to consider the many steps in the process, using flow charts like Figure 1, which is a block diagram of the steps in a typical GC method, with system thinking-related additions to reflect some of the external forces impacting the method.
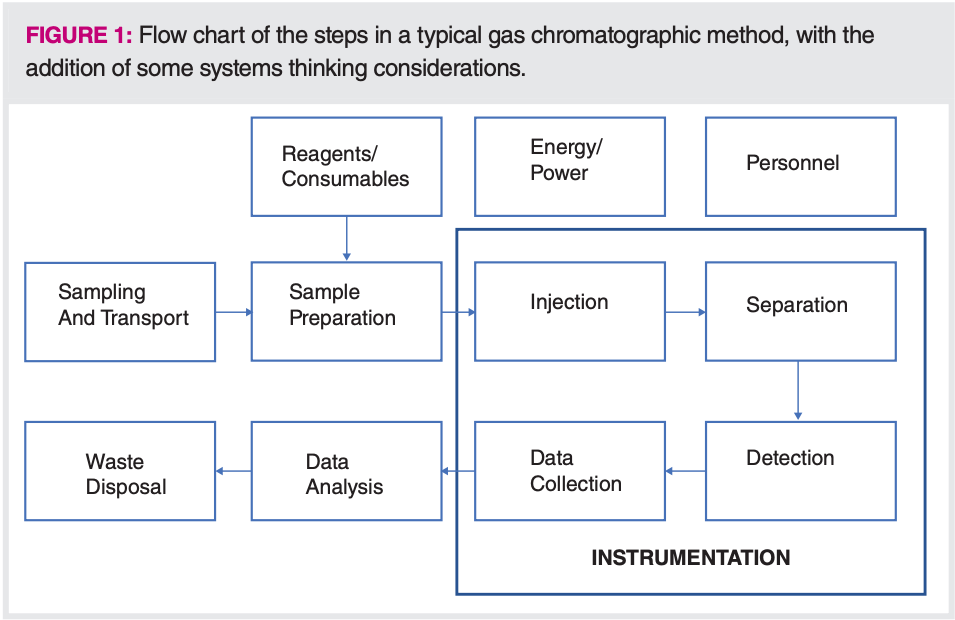
When optimizing the method, we think towards the overall goal of the analysis, and then optimize each step towards that goal. In addition to the usual analytical chemistry steps of sample preparation, instrumentation, and data collection, systems thinking teaches us to consider all related aspects of the method, including where the samples come from, how they are transported to the laboratory, the number of samples, reagents, and consumables, time and effort in data analysis, and where the samples, reagents, and materials go after the analysis is finished. Energy and personnel inputs are included in Figure 1 but not attached to a particular step in the method, as they impact all steps.
An example of an unintended consequence that systems thinking might expose could be seen in a switch from liquid–liquid extraction (LLE) to solid-phase microextraction (SPME) for the sample preparation, an obvious choice for making a GC method greener. LLE generally has the advantages of simplicity and low cost, with disadvantages of high solvent utilization. SPME, while clearly greener, may have higher costs, depending on fibre lifetime. More interestingly, equilibrium in LLE is generally rapidly achieved due to intimate and complete mixing of the two liquid phases, while SPME may require longer equilibration or heating of the extraction vessel, both of which might negate some of the green benefit. Longer equilibration time might then slow down the chromatographic analysis, causing the instrument to idle for longer time between runs, further reducing the benefit. Systems thinking and thinking holistically are therefore a critical part of truly making any analytical method greener.
A further aspect of systems thinking places the analytical method into the context of the whole life cycle of the product or process being measured. Systems thinking assists with the identification of bottlenecks or delays in processes that can be remedied, much in the way that chemists define the rate determining step in a reaction mechanism. Systems thinking underlies the current attempts to evaluate analytical and chromatographic methods for greenness. As we will see below, evaluating a method involves examining the sustainability aspects of each step in the process and all materials used. Two methods for evaluating the greenness of analytical methods are discussed below.
Analytical Method Greenness Score (AMGS)
The Analytical Method Greenness Score (AMGS) was proposed by Hicks and associates in 2019 (11). This metric provides a tool especially suited to chromatographic methods, and was demonstrated for high performance liquid chromatography (HPLC), ultrahigh-pressure liquid chromatography (UHPLC), and supercritical fluid chromatography (SFC). Its main focuses are on the mass of solvents used for sample preparation, instrument operation, health and environmental measures for the solvents, energy utilization for both the solvents and instrument, and the number of sample injections expected, including standards. In short, AMGS is related to instrument energy, solvent energy, and solvent safety. The authors note that energy usage is a major contributor to the greenness of any analytical method, and that this can vary widely, depending on the application and technique. They note that chromatographic systems integrated with additional instruments, such as a mass spectrometer, have significantly higher energy usage than the chromatograph alone. I further note that hyphenated techniques also may require a more specialized and expensive operator.
RGB Additive Colour Model
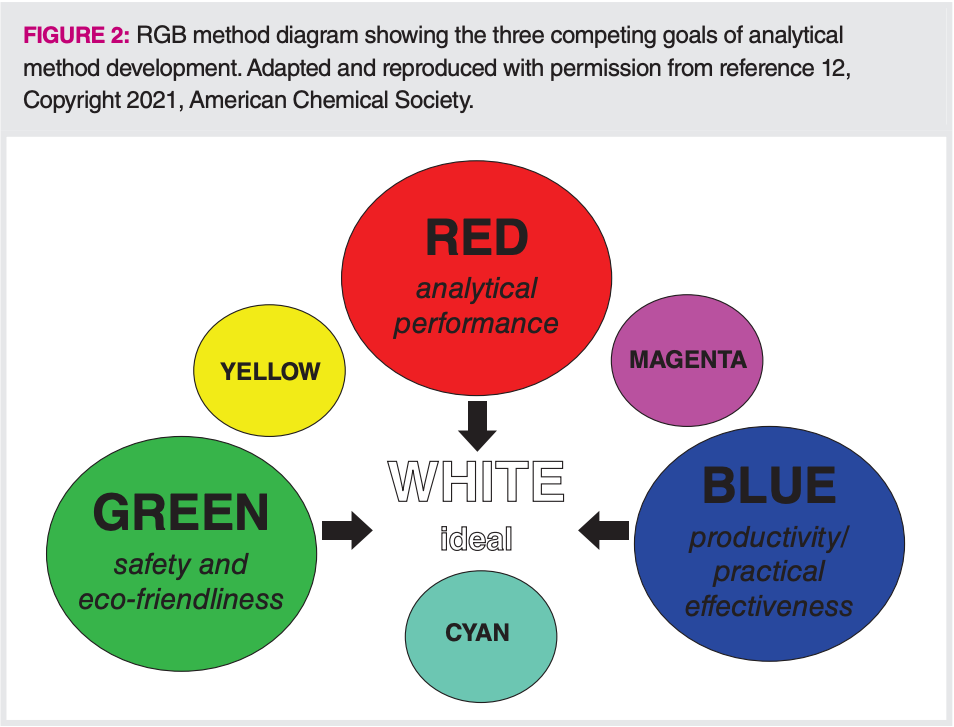
The RGB Additive Colour Model was developed by Nowak and Koscielniak for evaluating analytical methods according to analytical performance (red), productivity and effectiveness (blue), and safety and eco-friendliness (green), as seen in Figure 2 (12). Methods are given a numerical score from 1–100, based on how effectively they address each of these method goals. An interesting question about using this idea for method development or optimization lies in whether the three colours are competitive or complementary. Do productivity and performance have to be sacrificed to gain safety and eco-friendliness? Do practical methods sacrifice performance and greenness? Do high performance methods, such as those with extremely low detection limits, have to sacrifice practicality and greenness? These questions still require answers.
Making GC More Sustainable
There are several approaches that can make GC more sustainable, some of which we have discussed in recent instalments, including miniaturization and carrier gas choice. We discussed a sample preparation example above.
In an earlier instalment, we discussed several configurations and principles for smaller gas chromatographs that can perform the routine tasks generally done on larger systems (13). Today’s miniature gas chromatographs can perform all of the tasks necessary for most routine analysis applications. The sustainability advantages include smaller laboratory footprint, less heat generation and lower power consumption, and potentially simpler operation. One sustainability challenge in implementing miniature instruments is that replacing the existing instrument itself has up-front capital and sustainability costs that must be considered.
For several reasons, including high inertness, excellent column performance, slow peak broadening and, until recently, ready availability, helium is the most widely used carrier gas for capillary GC. Today, ongoing helium shortages are wellknown, and result from helium being obtained as a by-product of fossil fuel production (14). From a sustainability perspective, helium is probably the worst choice for carrier gas. Mainly on cost and availability, but now with an eye towards long-term sustainability, hydrogen and nitrogen are now more widely considered as carrier gases.
As a replacement for helium, hydrogen is an obvious choice, and has been discussed in the literature almost since the inception of capillary GC. From a chromatographic perspective, hydrogen offers faster separations and greater separation efficiency at high flow rates than either helium or nitrogen. From our sustainability perspective, hydrogen requires either the purchase of high pressure cylinders or a hydrogen generator, which is an additional capital expense items that may cost nearly as much as the gas chromatograph itself.
Nitrogen is classically considered the poorest choice of the three gases, from a chromatographic point of view. According to classical van Deemter plots, nitrogen rapidly loses efficiency as the flow rate is increased to practical values. However, in temperature programming, the effects noted in van Deemter plots do not apply. In a previous instalment, we discussed how nitrogen can provide very similar chromatographic performance to helium in many temperature programmed situations (15). Nitrogen is also more difficult to use with GC–mass spectrometry (MS), as it is slightly ionizable at the usual 70 eV used in electron ionization ion sources, so its signal can overload a mass selective detector. For the best combination of greenness and chromatographic performance in capillary GC, I recommend nitrogen as the carrier gas, followed by hydrogen (if nitrogen use is not possible), and then helium.
Conclusions
Green and sustainable chemistry are here to stay. As we move forwards in analytical science and chromatographic method development, principles of green chemistry and sustainability will guide our work. Applying principles of green chemistry to analytical methods will have the added benefit of making them more rugged, robust, and reproducible, while reducing waste, consumption, and cost. I encourage gas chromatographers to begin embracing green chemistry by examining their own methods, and considering sustainable changes, such as using nitrogen carrier gas, smaller, less power consuming instruments, and solvent-limited or solvent-free sample preparation.
Acknowledgement
The author’s work on green gas chromatography is supported by a grant from the American Chemical Society Green Chemistry Institute’s Pharmaceutical Roundtable and collaborations with Seton Hall University’s Academy for Green Chemistry, Sustainability, and Stewardship.
References
(1) Anastas, P. T.; Warner, G. C. Green Chemistry Theory and Practice; Oxford University Press, 2000.
(2) Lancaster, M. Green Chemistry An Introductory Text; 3rd Ed. Royal Society of Chemistry, 2016.
(3) American Chemical Society Green Chemistry Institute, https://www.acs.org/greenchemistry.html (accessed 2023-04-14).
(4) United Nations Sustainable Development Goals, https://sdgs.un.org/goals (accessed 2023-04-14).
(5) Snow, N. H. Selectivity and Sensitivity: The Electron Capture Detector (ECD), Its Unique Inventor James Lovelock (1919– 2022), and GAIA, LCGC North Am. 2022, 40 (11) 533–535, 542. DOI: 10.56530/lcgc.na.dm6277r4
(6) Carson, R. Silent Spring; Houghton-Mifflin, 1962.
(7) Lovelock, J. GAIA, A New Look at Life on Earth; Oxford University Press, 2016.
(8) Nowak, P. M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAc Tends. Anal. Chem. 2021, 138, 116223. DOI: 10.1016/j.trac.2021.116223
(9) Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAc. Trends Anal. Chem. 2013, 50, 78–84. DOI: 10.1016/j.trac.2013.04.010
(10) Meadows, D. H. Thinking in Systems; Chelsea Green Publishing, 2008.
(11) Hicks, M. B. et.al., Making the Move Towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) Calculator. Green Chem. 2019, 21, 1816–1826. DOI: 10.1039/C8GC03875A
(12) Nowak, P. M.; Kościelniak, P. What Color Is Your Method? Adaptation of the RGB Additive Color Model to Analytical Method Evaluation. Anal. Chem. 2019, 91, 10343−10352. DOI: 10.1021/acs.analchem.9b01872
(13) Snow, N. H. Let’s Get Small: Powerful Gas Chromatography in Small Packages, LCGC North Am. 2021, 39 (3), 128–131.
(14) Pflum, M. NBC News, “The Fate of America’s Largest Supply of Helium is Up In The Air.” https://www.nbcnews.com/science/science-news/fate-americas-largest-supply-helium-air-rcna69309 (accessed 2023-04-14).
(15) Snow, N. H.; McCann, S. P; Handzo, B.; Rana, H. Go With the Flow: Thinking About Carrier Gas Flow in GC. LCGC North Am. 2020, 38 (3), 152–158.
About the Author
Nicholas H. Snow is the Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, and an Adjunct Professor of Medical Science. During his 30 years as a chromatographer, he has published more than 70 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction. Direct correspondence to: amatheson@mjhlifesciences.com


Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.












