Generous Results with MISER Chromatography
Merck's Christopher Welch spoke to Alasdair Matheson, editor of LCGC Europe, about high-throughput analysis using MISER chromatography and how it can boost your laboratory?s productivity

Merck's Christopher Welch spoke to Alasdair Matheson, editor of LCGC Europe, about high-throughput analysis using MISER chromatography and how it can boost your laboratory’s productivity
What is MISER Chromatography and when was this concept devised?
The acronym MISER stands for "multiple injections in a single experimental run". It's a form of high-throughput chromatographic analysis that we've been using here at Merck for about ten years. MISER chromatography has received quite a bit of attention since our recent publication on the technique(1).
MISER chromatography is essentially flow injection analysis (FIA), the only difference is that, on the way to a detector, the samples are passed through a chromatographic column, which provides minimally sufficient resolution of the peaks of interest, or for LC-MS applications, resolution of the components of interest from interfering substances.
Basically, MISER is a very simple way to perform high-throughput analysis so that the entire experiment resides in a single chromatogram, thereby simplifying data analysis and interpretation. MISER doesn't work for every scenario, but in certain cases it greatly speeds up and simplifies the task of high-throughput analysis.
Why is this technique becoming more important for chromatographers?
Over the last few years there has been a big push toward the adoption of platform technologies that are, in effect, 'solution engines' for certain classes of problems. At Merck, we have platforms for quickly finding the best reagents, additives and conditions to use in a particular chemical reaction or purification. A variety of different conditions are screened in parallel, which generates lots of samples that have to be analyzed and interpreted before a conclusion can be drawn from the experiment.
Consequently, researchers are becoming burdened not only with an ever increasing number of samples, but also with the need to organize the resulting data in a way that helps interpretation and decision making. MISER chromatography both speeds up analysis time, typically to less than 1 min for each sample, but also greatly simplifies interpretation of the results by eliminating the need for the traditional steps of peak integration, data transfer and graphing. With MISER chromatography, we like to say that the chromatogram IS the graph.
What advantages does MISER Chromatography have over other high-throughput techniques and what is unique about your approach?
The MISER approach is best suited for optimization workflows with large groups of samples containing different levels of the same principal components. We like to use it to support screening when we are trying to identify microplate wells where a particular product is being formed at high levels, or where an impurity is being reduced to very low levels. The use of mass spectrometry detection allows individual components of interest to be extracted from the MISER chromatogram, or MISERgram. This provides a very effective visual readout for the experiment, allowing the researcher to quickly identify promising conditions for follow-up studies.
This visual simplicity is similar to that offered by the 'old school' multiparallel planar separation techniques of thin layer chromatography (TLC) or slab gel electrophoresis (SGE). The MISER technique is especially well suited for 'needle in a haystack' screening scenarios, where only a very few 'hits' may be turned up in the analysis of hundreds of samples. In this scenario, hits easily stand out from the surrounding misses. The technique doesn't work well for situations where very small differences in composition between samples must be measured, or where exact quantification is needed. The technique is also poorly suited to high-throughput screening approaches in which a different molecule of interest appears in each well, which is often the case in drug discovery. When it is possible, the speed and simplicity of the approach are so compelling that one gets used to designing experiments in such a way that MISER analysis can be used.
Can you illustrate with a practical example the benefits of this approach to a working chromatographer?
Sure, here's a recent example from our laboratories. We screened several hundred samples looking for conditions to perform the required reaction. All wells start out with the same amount of starting material, and varying amounts of product are formed in each well depending on the presence of the appropriate catalysts, additives solvents, etc.
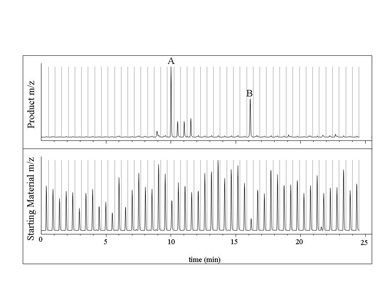
The MISERgram shows the analysis of a block of 48 samples (Figure 1). Mass spectrometry (MS) detection allows the extraction of relevant ions for both starting material and product. Most wells are unsuccessful, forming essentially no product, even though the starting material is being, at least, partially consumed. Several wells where product has been formed readily stand out, marked A and B. At this point, the researcher could confidently conclude that these two conditions deserve a closer look, perhaps following up with confirmatory analysis using conventional HPLC. It is probably also safe to conclude that well A is of greater interest than well B because this sample contains both more product and more residual starting material than well B — suggesting the possibility of additional product formation with prolonged reaction time.
For this approach to work, you need to make sure that the peaks of interest are not co-eluting with interfering substances. In this type of screening we often include organic bases like triethylamine in some wells. Not surprisingly, this can wreak havoc when these additives co-elute with our components of interest, causing significant ion suppression. The problem is easily overcome in most cases by adjusting the eluent strength such that the compounds of interest are retained, while the interfering substances are eluted at the void.
What sectors is MISER Chromatography currently being used in and for what applications?
We use MISER HPLC-MS for high-throughput analysis of samples coming from the screening of catalysts, enzymes and purification media. We sometimes use MISER LC-ICP-MS for high-throughput analysis of metals to support the screening for conditions to remove metal impurities. MISER chiral HPLC is sometimes used to support screening for enantioselectivity. MISER achiral HPLC with circular dichroism (CD)detection is can also be used for this application.
Recently, academic collaborators have also begun using the approach. I think the simplicity and flexibility of the technique and the fact that it can be performed on standard HPLC equipment make it a good choice for academic laboratories just breaking into the high-throughput experimentation game. Our Tet. Asymmetry paper contained an example of MISER analysis of caffeine in a variety of soft drink beverages, sports drinks, coffees and teas —potentially a fun laboratory project for an undergraduate laboratory course.
How will MISER Chromatography evolve in the future?
The first change that will be needed will be for instrument vendors to provide convenient software tools to allow multiple injections within a chromatographic run. We currently use Agilent LC-MS control software, and MISER chromatography is possible on some other instruments using injector programming.
At this stage, MISER is not possible with some instruments using standard control software, but this could be easily rectified. In principle some software algorithms that already exist in for example, preparative chromatography software could be used.
The algorithms for enabling multiple injections within a run are fairly simple, and have been a standard part of preparative chromatography software for many years. Hopefully, all HPLC and SFC vendors will adopt MISER enabling software features in the near future.
Improved software to visualize MISER results more clearly would be valuable.
It's very easy to pick out a single well of interest from among 12 or 24 injections, but mistakes in assignment are possible when looking at a MISERgram with 96 or more injections. It would be helpful to have software tool that would populate the individual peaks into a microplate viewer that would allow common scaling, zooming, etc. Such software would also help to simplify the interpretation of MISER runs involving overlapping chromatograms, for example, when a sample mixture with a two minute run time is injected every minute.
We almost always run MISER under isocratic elution conditions. It's possible to construct MISER gradient sequences, but this is not easily supported with existing instrument control software. In addition, short columns that can provide the minimal resolution required in MISER chromatography are needed. We currently use a variety of different guard columns for this application, but columns specifically tailored to this market can be imagined.
Finally, the cycle time for most of the MISER chromatography that we do is limited by the autosampler. Faster autosamplers will bring about a corresponding increase in throughput for MISER analysis.
How would you sum up MISER chromatography in one word?
Simplicity! MISER chromatography is a laboratory technique that speeds up and simplifies research. It's fundamentally a very simple idea, and, perhaps, just a subcategory of that long-established field of flow injection analysis. Nevertheless, the technique can be tremendously empowering. Sometime innovation comes in the form of bigger, more complex and more expensive equipment, and sometimes in comes in the form of simplicity. As researchers, we've got to get better at learning to recognize and implement the simple alternative when it presents itself.
Christopher J. Welch is the science strategy lead for global analytical chemistry at Merck Research Laboratories in Rahway, NJ, where he also chairs the organization within Merck that funds the acquisition and evaluation of new technologies. His research interests centre on the development and application of new separation and analysis technologies to aid the discovery and development of pharmaceuticals. Chris is a councilor for the American Chemical Society Division of Organic Chemistry and was recently named as an ACS Fellow.
Reference
- Christopher J. Welch, Xiaoyi Gong, Wes Schaefer, Edwin C. Pratt, Tanja Brkovic, Zainab Prizada, James F. Cuff and Birgit Kosjek, Tetrahedron:Asymmetry, 21(13-14), 1674-1681 (2010).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.








