GC–MS Analysis of Aroma Compounds in Edible Oils by Direct Thermal Desorption
GC–MS Analysis of Aroma Compounds in Edible Oils by Direct Thermal Desorption
Determining aroma compounds and off-flavours in edible oils by gas chromatography coupled to mass spectrometry (GC–MS) is routine in many food-producing companies; however, the oily matrix needs to be kept out of the analytical instrument to avoid impacting analytical stability. The following article describes a simple, yet efficient, way of eliminating the matrix while determining the flavour compounds in a sensitive and selective manner.
Photo Credit: VMJones/Getty Images

Products that taste good will be well liked and will sell well, but only as long as consumers are satisfied with the quality. This is nothing new, but it can be challenging for producers to ensure the uniform taste of products when raw products come from different sources, and different suppliers are used over the course of the year in different production locations worldwide.
Even though variations in flavour and taste rarely affect consumer safety, the offâflavours and aroma profile of a product can make a big difference in its acceptability. It is well known that consumers remember negative experiences more vividly than positive experiences - and that they tend to share a negative experience more readily with others. Therefore even the occasional negative eating experiences have a wide ranging and long lasting impact on future buying decisions. As a consequence of this producers are interested in frequent quality controls.
Sensory testing is traditionally performed to confirm an even product quality. This is very clearly a fully valid approach and we should not attempt to replace this with instrumental chemical analysis. However, the strength of instrumental analysis is that it can explain sensory findings and determine the reasons for flavour deviations. Analytical instruments also never get tired, are available at any time, and results are traceable to a calibrated balance.
In order to take advantage of the benefits of aroma analysis as a complementary tool to sensory testing, aroma specifications need to be established. These specifications require a reliable instrumental technique, which is robust and delivers correct results reproducibly. As aroma compounds very often are trace compounds hidden amongst a multitude of volatile matrix compounds, the analytical method of choice also needs to be quite sensitive. The selectivity of a GC in combination with a mass spectrometer is sufficient for most applications.
Determination of aroma compounds in edible oils (such as olive oil, sunflower oil, fish oil) is important for both manufacturers and vendors. Off-flavours derived from unsaturated fatty acid degradation are especially important (for example, hexanal, 2-[E]-nonenal and 2,4-[E,E]-decadienal), because these compounds can compromise the taste and therefore quality of a product even in the ng/g concentration range.1,2,3,4 Sensitive and fast analysis methods, ideally combined with simple sample preparation, are needed.
A sensitive analysis method based on dynamic headspace sampling was previously developed in 2008.5 At the time, direct thermal desorption from standard microvials placed in thermal desorption tubes was also tested, but although the technique performed well for volatile analytes, the resulting sensitivity for less volatile analytes was not satisfactory. In order to have good reproducibility, it is important to achieve high recovery of volatile aroma compounds such that the quality of the analysis results is not critically dependent on minor day-to-day variations in the analytical system. For very volatile and chemically stable compounds this is relatively easy to achieve. The difficulties arise with less volatile compounds. Therefore the focus in the development of any instrumental technique needs to be on these compounds. Generally speaking, it is desirable to minimize the distances in the system that the analytes must travel, minimize surface areas, and eliminate cool zones in the analyte flow path where these high boiling compounds may be lost through adsorption or condensation. In the case of this work, the oil samples were placed in microvials inside a thermal desorption unit for thermal extraction close to the site where the aroma compounds were subsequently trapped before being transferred to a GC system for analysis. In addition, the position of the slit in the microvials used to purge the analytes through to the trap was optimized for best recovery, that is, for most efficient purging of the analytes, minimizing the length of the flow path. In this project we returned to the microvial approach and evaluated different microvial designs to improve the transfer of high boiling compounds while maintaining the excellent performance for the volatile fraction.
Methods
Materials: Standard empty TDU tubes with a single notch were used for thermal desorption. Oil samples were placed in different types of microvials, in which purge slits were placed at different distances from the bottom of the microvial or which were cut to different lengths. Analyte recovery depends significantly on the distance between the surface of the oil sample and the purge slit. With increasing distance, the volume between the two is increasingly unswept by the purge gas. Analyte recovery is therefore reduced since it relies mainly on diffusion from the surface of the oil sample to the area near the purge slit.
Instrumentation: Thermal desorption of oil samples in microvials was conducted in a Thermal Desorption Unit (TDU) and analytes were refocused in a Cooled Injection System (CIS 4), PTV-type inlet (Gerstel) at a low temperature before being transferred to the GC column. A 7890/5975 GC–MS system (Agilent Technologies) was used for separation and determination of the analytes of interest. Thermal desorption tubes containing the samples were delivered to the TDU automatically using a MultiPurpose Sampler (MPS) (Gerstel).
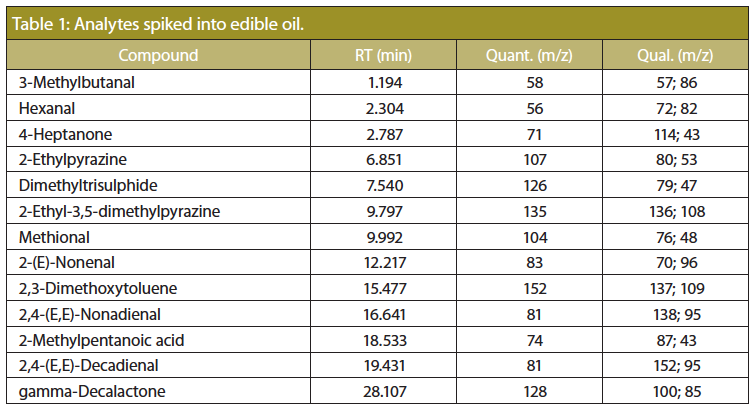
Sample Preparation: Edible oil was spiked with the analytes listed in Table 1 in the concentration range from 10 to 1000 ng/g. A set of 30 mg samples of the oil were weighed into individual microvials and placed in thermal desorption tubes. Each tube was capped with a transport adapter and placed in the autosampler tray for later desorption. The presented case focuses on edible oils, in this case of potato chip production quality. A range of aroma compounds was chosen, which cover a few relevant aroma attributes: methional (potato), pyrazines (roasted), alkenes (fatty/rancid, cardboard), 3-methylbutanal (malty), dimethyltrisulphide (vegetable, cabbage), and gamma-decalactone (creamy, coconut). For calibration purposes three internal standard compounds were added during sample preparation: one more volatile (4-Heptanone), one less volatile (2,3-Dimethoxytoluene), and finally 2-methylpentanoic acid as internal standard for volatile acids. These compounds do not occur naturally in food material and have excellent chromatographic properties making them well suited as internal standard compounds.
Analysis Conditions: TDU: solvent venting; temperature programme: 30 °C; 200 °C/min; 90 °C (15 min); PTV: glassbead liner; 0.2 min solvent vent (30 mL/min); split 3:1; temperature programme: -70 °C; 12 °C/s; 280 °C (30 min); column: 15 m × 0.25 mm, 0.25âmm ZB-FFAP (Phenomenex); pneumatics: He, constant flow = 1.3 mL/min; oven temperature programme: 35 °C (1 min); 4 °C/min; 120 °C (5 min); 50 °C/min; 250 °C (8 min); MSD: SIM, cf. Table 1.
Measurements: Ten oil samples of 30 mg each were prepared in microvials of the selected design and individually desorbed in the TDU to determine the repeatability.
Results and Discussion
Thermal extraction of aroma compounds from edible oils using microvials can prevent contamination of the analysis system by highâboiling matrix compounds while allowing effective transfer of semi-volatile organic compound (SVOC) analytes onto the analytical column. Thermal desorption of the aroma from the oil sample in the microvial should be performed at relatively mild temperature conditions to avoid both analyte degradation and the formation of additional aroma compounds by thermal degradation of the sample. On the other hand, the recovery of aroma compounds during thermal desorption increases dramatically with increasing temperature, especially for less volatile compounds. Depending on the nature of the sample, a good compromise has to be found. After analysis the microvial can be disposed of and the desorption tube is ready to take up the next sample.
Figure 1: SIM chromatogram resulting from thermal desorption of a spiked edible oil inside a microvial with slit placed 1 cm from bottom.
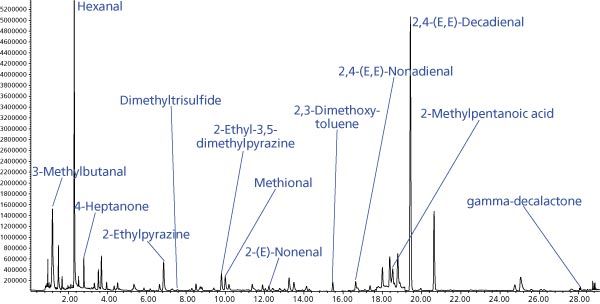
In our work, we determined that microvials with a purge slit placed at a height of 1 cm from the bottom were the most effective for analyte transfer, especially for high boiling compounds (gamma-decalactone), as shown in Figure 1. Analyte recovery expressed as relative peak area for the different types of microvials used are shown in Figure 2.
Figure 2: Comparison of analyte transfer from different types of microvials. Peak areas are normalized to the largest observed peak for the respective compound.
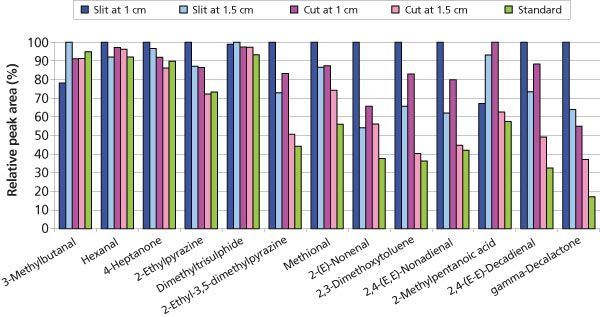
It is reasonable to assume that the vapour phase directly above the sample surface would be more efficiently purged when using the slit design than when using a standard microvial, which mainly relies on analyte diffusion through a stagnant vapour phase volume because of the extended distance between the sample surface and the purge flow path. In other words, a microvial with a slit enables faster and more efficient analyte transfer from the sample to the analysis system. A different effect seems to be responsible for the improved transfer of 2-methylpentanoic acid when using the microvial cut to a 1 cm length. The reduced glass surface area in the analyte transfer path could be beneficial for transfer of active compounds such as acids.
Figure 3: Relative peak area standard deviations resulting from thermal desorption of edible oil samples (n = 10) in slitted microvials (1 cm from bottom).
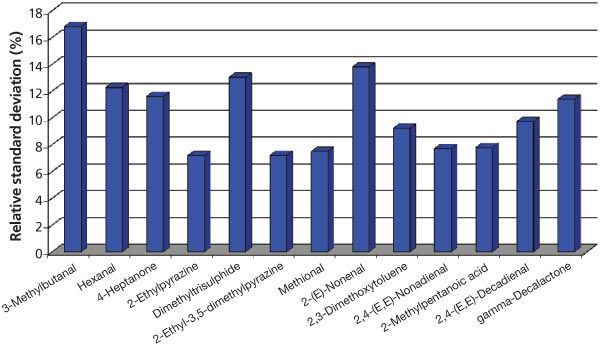
Relative standard deviations for 10 repeat measurements with the 1 cm slitted microvial were between 7.2% and 16.8% with a median of 9.7% (Figure 3). This is highly acceptable considering the complex matrix, the low concentrations, and the straightforward sample preparation. A longer desorption time would likely improve the relative standard deviations further.
Conclusions
- Aroma compounds can be determined in edible oil down to the ng/g concentration range by direct thermal desorption from a slitted microvial.
- Introduction of liquid samples to the microvials is easily performed using a pipette and is the only required sample preparation step.
- The microvial design with the slit at 1 cm distance from the bottom was found to deliver the best performance.
- Slitted microvials can be beneficial for the determination of volatile and semi-volatile compounds in other liquid, paste, or solid materials.
References
1. E. N. Frankel, Journal of Agricultural and Food Chemistry58, 5991 (2010).
2. E. Choe and D.B. Min, Comprehensive Reviews in Food Science and Food Safety5, 169 (2006).
3. X. Pan, H. Ushio, and T. Ohshima, Fisheries Science71, 639 (2005).
4. C. Karahadian and R.C. Lindsay, Journal of the American Oil Chemists Society66, 953 (1989).
5. O. Lerch and C. Gil, Gerstel App Note 03/2008.
Alexander Hässelbarth, Ph.D., is a specialist in flavour analysis and flavour technology. He has worked for nearly 20 years in various positions in flavour research at Kraft Foods, Germany. In 2011 he and Claudia Geyer founded FlavoLogic GmbH, a flavour consultancy in Munich, Germany. Together with his team, he provides solutions for off-flavour issues, aroma analysis, sensory testing, innovative flavour technology, and customized flavours and flavour recipes.
Oliver Lerch, Ph.D., works as a scientist in the Analytical Services department at Gerstel. After receiving his Ph.D. from the Ruhr University Bochum, Germany, in 2003, he worked as a postdoctoral fellow at the Max-Planck-Institute of Molecular Plant Physiology in Potsdam, Germany, before joining Gerstel in 2005.
E-mail:alexander.haesselbarth@flavologic.com
Website: www.flavologic.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







