Explaining Silyl Ether Formation (SEF) in Supercritical Fluid Chromatography (SFC)
The Column
Explaining Silyl Ether Formation (SEF) in Supercritical Fluid Chromatography (SFC)
As interest in supercritical fluid chromatography (SFC) grows, practitioners require that the technique delivers reproducible results. For SFC separations to be successful in regulated quality control and release testing laboratories, the robustness of the separation system must be well understood. The industry standard mobile phase in SFC separations is compressed CO2 mixed with a co-solvent, most often methanol, chosen for cost, speed, and solubility reasons. However, the water-free nature of CO2/methanol mixtures can impose slow changes to chromatography over time as a result of interaction between the stationary and mobile phase. These changes are documented and defined in this article.
Interest in supercritical fluid chromatography (SFC) has grown in recent years, in large part as a result of new chromatography products and stationary phases. New SFC-specific stationary phases now include bondings and ligands designed and optimized for use in CO
2
-based separations, which can reduce or eliminate the need for additives. One of the most compelling reasons to consider SFC over other separation modes is the green nature of CO
2
-based mobile phases (less organic waste and faster dry down). These mobile phases are also relatively inexpensive, offer exceptional solubility for small molecules and are particularly useful for performing preparative separations. In addition, SFC mobile phases are typically, or are nearly, water-free, which makes them ideal when separating analytes that are hygroscopic or degrade quickly in the presence of water. Also, SFC is particularly well-suited for the recovery of free bases. A common misconception with stationary phases is the assumption that the retentive surface never changes and that the interaction with the mobile phase is only to affect retention. The condensation of alcohols with silanols to form silyl ethers is a wellâknown and well-understood phenomenon in silica chemistry.
1
This phenomena occurs under the water-free conditions of SFC and is a result of the slow reaction of the alcohol co-solvent with silanols to create silyl ethers on the particle surface. We have recently shown that the changes in surface polarity arising from silyl ether formation (SEF) contributes to changes in retention and selectivity in SFC separation.
2
In this article, we describe the observable characteristics of SEF - in particular, changes in chromatographic results over time. While not every change in retention can be or should be attributed to SEF, our investigations reveal that this can be a significant contributor to variability. This type of slow, steady change in retention of SFC stationary phases has been documented.
3
Figure 1: An example equilibrium of methanolic alkoxylation, which can be pushed to the right-hand side in water-free conditions, resulting in methyl-silyl ethers. The slow condensation towards silyl ether formation (SEF) causes a chemical change to silica particle surfaces that is many times slower than the hydroxylation reaction.
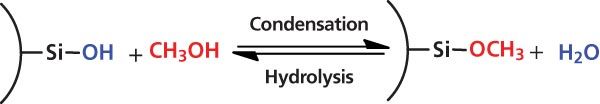
Found on the surface of silica-based materials, silanols react with alcohols (methanol, ethanol, etc.) in the mobile phase to create surface-bound silyl ethers. As shown in the equilibrium depicted in Figure 1, a silanol can be converted to a methyl-silyl ether to produce a water molecule. In simplistic terms, the surface is being bonded with ethers. Under typical SFC conditions, the equilibrium is driven towards the formation of silyl ethers. SEF should not be confused with physical changes to a packed bed structure, for example, voids or bed shifting, resulting in significant changes in peak profiles (loss of efficiency, increase of peak tailing). The change is chemical in nature and will affect the chromatography. Furthermore, the reaction equilibrium is greatly influenced by the presence of water, even in small amounts. Because typical SFC separations use water-free, or nearly water-free, mobile phases and silanols are commonly used to retain analytes, the effects of SEF are detectable. SFC is mostly operated in normal phase mode, which functions by using polar-polar interactions between analytes and the stationary phase. The polar surface of silica-based particles affects retention and selectivity under those conditions. Decreasing the polarity of the particle surface results in an obvious loss of retention and change in selectivity. In the past, normal-phase liquid chromatography (LC) had similar equilibration issues, often as a result of the presence of residual water. Practitioners worked around the problem by either using very dry solvents or saturating the mobile phase with water (that is, vigorous shaking of hexane with water before use). Some of the reluctance to use normal-phase LC, in favour of reversedâphase LC, can be attributed to these issues. For preparativeâscale separations, compound collection is often done in a mass spectrometry-directed fashion, so the change in retention or selectivity may be ignored.
Conditions for Detecting SEF
The SEF reaction requires two basic parameters: 1) that the liquid phase be nearly water-free, and 2) that the liquid phase must contain an alcohol. While SEF is prevalent under SFC conditions, the chromatographic response will not be observed in all modes of chromatography. For example, size-exclusion chromatography (SEC), reversed-phase LC, and hydrophilic interaction liquid chromatography (HILIC) separations make detection of SEF unlikely because of the nature of the mobile phases and the separation mechanisms used. When performing non-retentive separations (for example, SEC) there is little or no interaction between the analyte and stationary phase surface (well chosen SEC phases will prevent adsorption of analytes to the particle surface). For a silanol and alcohol to condense, the subunits must be in proximity and the reaction must be favourable. Other nucleophiles are expected to react in a similar fashion with silanols, but alcohols will form the defined silyl ethers. Without a catalyst, the reaction is quite slow, and can occur continually for several days. We have successfully catalyzed SEF reactions on a chromatographic column by mixing a tertiary amine into methanol and observed an acceleration in the retention drift effect.
2
In the case where a reversedâphase LC material or, more generally, an endcapped stationary phase is used in SEF favorable conditions, we have observed an increase in retention over time. The retention increase is not a result of SEF, but can be explained as a gradual loss of bonded phase, exposing silanols, which increases retention of hydrophilic analytes. We speculate that it would be possible to chromatographically detect SEF under HILIC conditions, where the weak eluent is acetonitrile and the strong eluent is methanol. However, confirmational testing has not been attempted and those conditions are rarely used. When there is a significant amount of water (>1% by volume) in the mobile phase, the equilibrium will shift towards the free silanol form, which is nearly always the case for reversed-phase LC and HILIC separations. Detecting SEF on chiral stationary phases used under SFC conditions is more difficult. The most popular chiral phases are made of wide-pore, low surface area (about 10 times less surface area than comparable smaller pore materials) silica particles that have been thickly coated or functionalized with polysaccharides. The chiral separation mechanism takes place in the chiral environment afforded by the high order structure of the polysaccharides - very different from the interactions of small-ligand bonded phases. It has been well-documented that these phases are susceptible to changes when exposed to strong acids or bases,
4
which is related to changing the chemical nature of carbamateâlinked selectors, but not the silica surface. SEF is not expected to play a significant role or be detected in polysaccharide-coated or functionalized phases because of limited access to silanols. In the case of bonded chiral selectors (brushâtype), if the phase has accessible silanols (not endcapped), SEF is expected to occur and retention changes would be detectable. If a brush-type chiral phase is endcapped, increases in retention over time could be observed as endcaps hydrolyze from the surface.
Explanation of Evidence and Mitigation Routes
We have documented both chromatographic and nuclear magnetic resonance (NMR) evidence of the formation of silyl ethers on both bonded (2-ethylpyridine) and unbonded silica (and inorganic/organic hybrid silica
5
) particle surfaces.
2
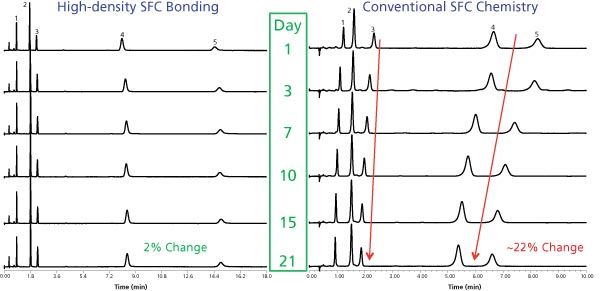
Our chromatographic findings allowed us to learn more about the SEF process, such as the relative reaction speeds on silica and hybrid silica particles; the latter being about two times slower because of lower silanol activity and not as a result of the number of silanols (stoichiometrically there are only ~10% fewer silanols). Figure 2 is an example of the retention change difference between a traditional SFC stationary phase and a modern phase that uses a high-density bonding to mitigate the effects of SEF. This is accomplished through limiting access of analytes to silanols on the particle surface. Even though SEF is still believed to occur at the particle surface, retention over time is unaffected because of stronger interaction of the analytes with the polar ligand.
Figure 2: Mitigation of retention drift caused by SEF. In typical SFC conditions, conventional silica-based SFC columns undergo particle surface changes resulting in reduced retention over time. Chromatographic conditions: columns: 3.0 × 100 mm; flow rate: 1.5 mL/min; mobile phase: 90/10 CO2/methanol; temperature: 35 °C; pressure: 2500 psi ABPR. Compounds left to right: 1. Papaverine; 2. Fenoprofen; 3. Prednisone; 4. Sulphamethoxazole; 5. Sulphanilamide.
Our experiments show that a column exhibiting lower retention as a result of SEF can be returned near to its initial retention state by flushing with many volumes of water. Furthermore, we successfully baseâcatalyzed SEF and hydroxylation using hybrid silica particles, as monitored by the change in selectivity of a pair of well-chosen analytes. We were also able to form silyl ethers with different alcohols, ethanol and n-propanol. We found the reaction rates to be many, many times slower than that of methanol, but the chromatographic response was similar. These results all point to a characterizable chemical reaction taking place on the particle surface, which has a detectable effect on the chromatography. In our investigations, we have not been able to attribute significant changes in peak shape to SEF. None of our evidence suggests that the acidity or reactivity of CO
2
enhances or impedes SEF in CO
2
/methanol mobile phases, because we have performed the SEF reaction in pure methanol mobile phases; only the exposure time and frequency or concentration of methanol had an effect. As a final note, SEF can be mitigated by doping mobile phases with water; however, the controllability and reproducibility is questionable.
Conclusions
We have identified changes in surface chemistry resulting from SEF as a significant source of retention drift in SFC when using alcohol modifiers under non-aqueous conditions. This observation is important to users of SFC because the gradual decrease in retention times can result in chromatographic irreproducibility for a given method.
References
1) C.J. Brinker, J. Non-Cryst. Solids100, 31–50 (1988).
2) J.N. Fairchild, D.W. Brousmiche, J.F. Hill, M.F. Morris, C.B. Boissel, and K.D. Wyndham, Anal. Chem. 87, 1735–1742 (2015).
3) K. Ebinger and H.N. Weller, J. Chromatogr. A1332, 73–81 (2014).
4) J. Putnam and G. Guiochon, J. Chromatogr. A 1216(48), 8488–8495 (2009).
5) K.D. Wyndham, J.E. O’Gara, T.H. Walter, K.H. Glose, N.L. Lawrence, B.A. Alden, G.S. Izzo, C.J. Hudalla, and P.C. Iraneta, Anal. Chem.75, 6781–6788 (2003).
Jacob Fairchild is a senior research scientist at Waters Corporation in the Consumables Business Unit (CBU). After receiving his PhD from the University of Tennessee in 2010, he has been researching and developing novel SFC stationary phases. The research team at Waters has a goal to understand selectivity in CO2-based mobile phases and improve the relative ruggedness of columns.
Darryl Brousmiche is a principal scientist in the CBU Synthesis Group at Waters Corporation. He is involved in the development of new stationary phases, SPE materials, and tagging reagents.

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








