Future Needs of HPLC and UHPLC Column Technology
LCGC Europe
In his final “Column Watch” instalment, Ron Majors looks into his crystal ball and discusses future needs in the areas of high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC) column technology and related instrumentation.
Ronald E. Majors, Column Watch Editor.
In his final “Column Watch” instalment, Ron Majors looks into his crystal ball and discusses future needs in the areas of high performance liquid chromatography (HPLC) and ultrahigh-pressure liquid chromatography (UHPLC) column technology and related instrumentation. He looks at where current technology may be heading and makes a prediction that monolith-based columns may still have a rightful place in the HPLC and UHPLC laboratory. This article concludes Majors’ legendary tenure as a monthly columnist for LCGC.
Next month, high performance liquid chromatography (HPLC) will celebrate its Golden Jubilee - 50 years of solving separation and analysis problems in just about every realm of science. Since the beginning, there have been major advances in the technology from the separation columns to the instrumentation to the data analysis and reporting. Large-particle packings of 50-µm diameter have given way to microparticles with diameters smaller than 2 µm, HPLC systems have gone from constant gas pressure pumps operating at 1000 psi to pumps capable of 20,000 psi pressure, detectors have progressed from simple 254-nm UV detectors to several hundred thousand dollar high-resolution mass spectrometers, and data output has moved from strip-chart recorders to high-speed computers with the ability to handle complex chromatograms. Constant improvements have been made along the way and should continue into the future. Bear in mind that chromatographers are, for the most part, rather conservative individuals who are not necessarily adopting improvements as soon as they are shown in the research laboratory. In fact, I have observed that it takes nearly a decade for a new column technology to become commonplace in the chromatography laboratory, not just because of conservative chemists but also partly the result of requirements for regulated methods, instrumentation keeping up with the column technology, and the time it requires to take column manufacturers to make the investment to transfer the technology from the research laboratory to the manufacturing floor.
Where Are HPLC and UHPLC Columns Heading?
For nearly 33 years in LC and LCGC magazine, I have been reporting on developments in HPLC column technology. I would now like to look into my crystal ball and attempt to focus on areas where further improvements in column technology may be needed. Continued investment in column technology is bound to continue since the HPLC and ultrahigh-pressure liquid chromatography (UHPLC) columns market has been very strong; otherwise there wouldn’t be around 200 companies involved in some aspect of the business. Columns are considered a consumable. An average instrument uses seven columns per year (1) so as the number of instruments grows, so does the columns market. The overall market for columns (analytical, preparative, capillary-nano, packings, and column accessories) is now estimated to be just over a billion dollars with an overall growth of 3.5%, but a higher growth in the UHPLC segment (2).
As pointed out earlier, tremendous strides have been made in particle and stationary-phase technology over the years. However, user demands in industry for productivity and sensitivity improvements continue to push further development in columns that are more efficient, faster, and more inert. These needs stretch from the research laboratory and through all phases of development up to manufacturing and quality control.
Recently, the 2.6-2.7 µm superficially porous particle (SPP) columns have established themselves as the favoured column type for new method development compared to the sub-2-µm totally porous particle (TPP) columns. The SPP columns provide lower pressures, higher or equivalent efficiency, and nearly the same loadability (3). In last year’s Pittcon report (4), SPP column introductions outnumbered TPP introductions 10:1. It has been shown that methods developed on older porous particle columns can be switched to these newer column types with minor adjustments. Regulatory bodies are already blessing these new column types. The new breed of SPP columns may actually break the 10-year cycle in adoption delay!
Over the next few years, expectations are that column manufacturers will continue to exploit this technology by filling out their SPP offerings with stationary phases that chromatographers apply to their everyday separations as well as for specialized separations, such as chiral compounds and biological pharmaceuticals. Sub-2-µm SPPs are already available and more are envisioned in the future. Larger particle SPPs in the 4-5 µm range are displacing established methods based on the popular 5-µm TPP columns. Wide-pore SPP columns have been developed for the efficient separations of large biomolecules.
Figure 1: Comparison of pore structure and diffusion paths of SPPs prepared by different processes: Particles made by (a) the multilayer coacervation method and (b) the PMT method. (Courtesy of Bill Barber, Agilent Technologies).
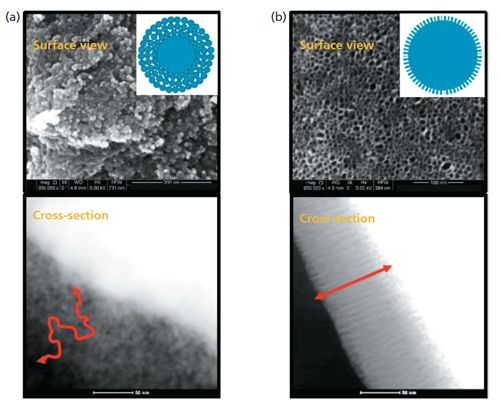
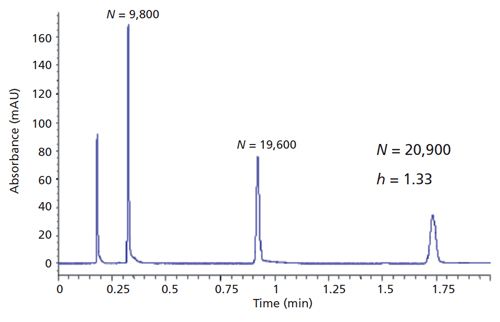
Even with all of the current improvements in column particle technology, work is continuing on further improvements in SPPs. Current techniques for making SPPs are based on a multilayer process or a coacervation process (5), and most commercial SPPs use one of these two processes. There are alternatives being investigated that result in improved TPP and SPP materials by allowing the formation of more-uniform mesopores compared to present packings. One pore-formation process is termed micelle-templating; in this process, mesoporous silicas are synthesized in the presence of cationic or nonionic surfactants to form highly uniform pores (6,7). A second process for making superficially porous micelle-templated particles is by direct synthesis (8,9). A third process termed micelle-templating with pseudomorphic transformation (PMT) has been used to make SPPs that also have a uniform porous structure (10). Without getting into the details of the actual patented process, Figure 1 provides the resultant difference in structures between current SPPs and one micelle-templated SPP produced using the PMT process. As you can see from this figure, the uniform pores are normal to the surface, contain a thinner shell with a high surface area, and result in a smooth surface with a uniform particle size. As can be seen in Figure 2, a 1.8-µm PMT SPP column shows excellent efficiency, roughly a 50% increase compared to TPPs of the same particle size, column dimensions, and chromatographic conditions.
Figure 2: Chromatographic results obtained using a 1.8-µm PMT SPP column. Column dimensions: 50 mm × 4.6 mm; mobile phase: 55:45 (v/v) acetonitrile–water; flow rate: 2.0 mL/min. (Courtesy of Bill Barber, Agilent Technologies).
Further investigations into TPPs are continuing. With all the discussion about improvements in SPP column performance based on the narrow particle size distribution of SPPs and how it affects the van Deemter A term, Supelco chemists looked back at sub-2-µm TPPs with equally narrow particle size distributions and noted an increase in chromatography efficiency by this change alone (11). Despite all the advances in SPP technology, there are still many more TPP stationary phases covering the particle size range from 1.5 µm to 10 µm so users presently have a wider choice of columns to use for analytical to preparative scaleup.
Don’t Count Monolithic Columns Out Just Yet
Monolithic columns have been around for a couple of decades. They have the advantages of being a single particle in a column with large macropores through which solvent can easily flow and with mesopores where the stationary phase can interact with the solutes coming through the column. The macropores give very low pressure drops and most of the commercial monoliths can be used with conventional 400 bar HPLC systems. Silica monoliths were introduced in 1998 and commercialized in 2000. Because of intellectual property concerns, they were mainly commercialized by one company and thus there was little incentive for other manufacturers to work on improving the technology. Now that patent protection is going away, there may be more interest in pursuing this technology. These monolithic columns, both silica- and polymer-based, still have great promise if researchers can figure out how to improve the efficiency without great increases in back pressure, make them in longer lengths needed for difficult separations, and contain them in a suitable housing to withstand the high pressures for very long columns. Silica-based monolith columns have low pressure drops, even lower than SPP columns with the same column efficiency. The first generation of silica monoliths had the efficiency of a 3.5-4 µm silica. The second generation has the efficiency equivalent to 2.0-2.3 µm silica albeit at a higher pressure drop (>2× higher for the same chromatographic conditions) because of the change in the macropore-mesopore domain ratio. For many years, only bare silica and C8 and C18 bonded phases were available, but now a few more stationary phases have become commercially available. Capillary, analytical (2.0- and 4.6-mm i.d.), and preparative 25-mm i.d. columns are available.
Polymeric monoliths, which are less covered by patent issues, could be quite attractive since their wider pH operating range gives them some advantages compared to silica monoliths, but the efficiencies of research polymeric columns still don’t live up to the commercial silica monoliths. A significant advance in polymeric monoliths has been the ongoing research work in their application to smaller molecules. In the past, polymeric monoliths were considered to be suitable for large biomolecules only. Novel approaches to make polymeric monoliths more appealing haven’t resulted in commercial products that can be used by practicing chromatographers. Monoliths may become the favoured approach for laboratory-on-a-chip systems since they can be synthesized in situ inside the narrow channels where efficient packing of particles has proven exceedingly difficult.
When comparing the performance of various types of HPLC and UHPLC columns (see later section), if monolith columns can be improved to fulfill the three criteria mentioned above, in the long run, they may prove to be the best approach for difficult separations needing many theoretical plates but with longer analysis times. The containment issue with silica monoliths will be a continuing challenge as long as the silica rods have to be made outside of a high-pressure column enclosure. Perhaps a return to the old radially compressed column concept would be one way to provide higher pressure and longer monolith columns.
Instrumentation Improvements Needed to Support Further Column Development
Instruments have been trying to keep up with column developments. Obviously, the life cycle for instrument development is much longer than what is required for new packings and columns. An area where it is well recognized as a hindrance to exploiting further improvements in column efficiency is the instrument contribution to band dispersion attributed to the HPLC and UHPLC instruments and their column-instrument interface designs. There is no doubt in my mind that instruments will see further improvement in lowering band dispersion to handle smaller SPPs. What is needed is a closer integration of the column hardware and instrument connections such that dead volumes may be almost nil, much like what has been achieved in some nano and chip instruments. The area of frit and endfitting design needs attention since the column packing where the separation actually takes place should be located at or near the injector device and the detector measurement device. This may necessitate a new column design that not only cuts down on this extracolumn volume, but also can withstand the higher pressures anticipated with smaller SPPs. Such designs are within the capabilities of engineers at the instrument companies but may necessitate the development of proprietary interfaces that may rule out the ability of the end user to select a column of their choice. Getting universal agreement among the instrument companies on a standard zero-dead volume interface would probably be next to impossible given the competitive environment that currently exists within the community. Perhaps some sort of cassette system without the typical compression endfittings, similar to what has been used in some commercial chip-based column configurations, might be used advantageously for closer coupling and easy, rapid column replacement.
As far as instrument pressure capability, UHPLC systems can be built to go to even higher pressures since pumps capable of thousands of bar (tens of thousands of psi) are already available for industrial use and chromatography engineers would have to adapt some of the same operating principles to achieve pulseless flow control in the microlitre to millilitre per minute range at pressures up to 100,000 psi. If SPP columns continue to dominate in the future, there may not be a need to greatly exceed today’s pressure limits. However, in chromatography pressure is always a useful commodity.
Miniaturization of Columns and Instruments
Small internal diameter columns in the capillary area (0.1-0.3 mm i.d).and nano area (less than 0.1-mm i.d.) are readily available, but their long-term column stability relative to larger bore analytical columns has been questioned and column efficiency is not as good as typical large-bore analytical columns. A lot of these problems have to do with the lack of adequate packing techniques for small internal diameter columns, in general. More attention should be paid to this aspect of column technology.
Further miniaturization of standard UHPLC instrumentation is possible. Microfluidics has already proven to be an alternative approach to perform analytical separations. Such downsizing of the LC experiment would certainly require a major redesign in the column and instrumentation. The use of miniaturized instruments would result in significant solvent, bench-space, and sample savings, and with mass spectrometry (MS) would allow even better interfacing. Chip-based LC systems have been investigated extensively, and a limited number of instruments have already been introduced to the market. However, the adoption rate for commercial instruments has been rather slow and, compared to regular analytical columns, in microfluidics column efficiencies are not as high as expected. Nevertheless, the significant advantages provided by miniaturized LC systems may spur further commercial development beyond the academic environment. One of the bright spots in column technology adaptable to microfluidics-based systems is the potential for the synthesis of in situ stationary phases via monolith formation. The packing of microparticles within narrow channels is difficult, and one of the reasons for low column efficiency in microfluidics-based column systems.
How to Compare HPLC Column Performances
How does one know if a particular column is the best that can be chosen? Table 1 shows the variety of common methods used to compare the performance of HPLC and UHPLC columns. Some of them are well known, but the ones that provide the best comparison take into account efficiency, analysis time, and pressure drop - three of the main chromatographic parameters that users are interested in for their analysis. The Poppe plot favoured for a number of years has been refined and is now supplemented by the kinetic plot. Developed by Professor Gert Desmet and colleagues at the Free University of Brussels in Belgium (12), the kinetic plot can be constructed by converting van Deemter plots to those based on a tR versus N plot, where tR is solute residence time (here defined at 10 times the column void time, t0), and N is the number of theoretical plates. The kinetic plot can compare the performance of different length columns and different stationary phases and compare the analysis times needed to achieve a certain level of efficiency at a maximum instrument pressure. For example, with the kinetic plot one can look at practical constraints on column length and particle size to choose an optimum configuration for the needed efficiency or analysis time.
Figure 3: Comparison of kinetic performance (residence time of analyte eluted at 10 times t0 versus maximally achievable plate number at the given permissible pressure) for a series of state-of-the-art fully porous particles (red data), core–shell particles (blue data), and silica monolith columns (black data) used in isocratic reversed-phase LC analysis of small molecules using acetonitrile–water mobile phases. The dashed black data lines represent the hypothetical performance of the silica monoliths if they could withstand a pressure of 1500 bar. (Adapted with permission from references 14 and 15.)
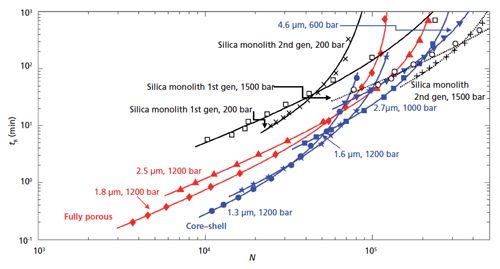
Desmet and his colleagues Cabooter and Broeckhoven have done an actual experimental comparison of three types of popular columns using the simplified kinetic plots shown in the busy Figure 3 (13) based on data from references 14 and 15. The figure shows the following column types: fully porous particles (red), core-shell SPP (blue), and silica monoliths, first and second generation (black). In addition, they added hypothetical silica monoliths (first and second generation) that would be capable of withstanding a pressure of 1500 bar (black data in the upper right). Note that current silica monoliths are operational at pressures as high as 200 bar because of the polyether ether ketone (PEEK) housing that encompasses the silica rod.
The lower left side of the kinetic plot is where one would focus to find the best column for a simple separation requiring a few thousand theoretical plates and an analysis time of a few minutes at most. The upper right hand side of the plot would be best if the user has a very complex sample and needs a lot of theoretical plates and has time on their hands to realize these large number of plates. According to the plot in Figure 3, in the simple case, the 1.3-µm core-shell SPP run at a pressure of 1200 bar would win out because it provides the greatest number of plates in the shortest time. However, a TPP of 1.8-µm diameter operating at 1200 bar would give the SPP a run for its money timewise, but only provide a fraction of the theoretical plates for the separation. The present silica monoliths shown in black in the top middle of the plot do not provide very good kinetic performance since they are limited to a pressure of 200 bar. However, as shown by the black lines in the upper right of the plot, because of their lower pressure drops and good efficiency, if a new generation (hypothetical right now) of silica monolith could be developed to stand up to high pressure (1500 bar) and be able to provide a high number of theoretical plates by an increased column length, they would win out for complex, multicomponent separations and would have a rightful place in the chromatography laboratory. Thus, the work is cut out for the monolith proponents to come up with that new phase and an appropriate column hardware.
Other Areas of Needed Improvements in Column Technology
Biochromatography Columns: As biopharmaceuticals such as monoclonal antibodies and peptide-based compounds continue to make inroads in the drug market, columns capable of providing high recovery separations of biologically-derived compounds, oligonucleotides, and biosimilars, both neat and in biological fluids, will be in big demand. Column manufacturers are already responding with biocompatible columns that provide more selective separations with higher recovery. Oligonucleotide purity requires columns that separate a wide range of oligomers, sometimes at high pH, so chromatographers in that field are always on the lookout for high efficiency, high-pH-tolerant columns. Biochromatographers will need to have columns that cover most of the HPLC modes including reversed-phase, ion-exchange, aqueous size exclusion, affinity, normal-phase, hydrophilic interaction, and hydrophobic interaction chromatography. The main requirement, of course, is that the pore size of biochromatography packings must be large enough to accommodate the largest biomolecules encountered.
Column Specifications and Features: Columns that are more inert and provide symmetrical peak shape will always be in demand. In the past couple of decades, stationary phases have come a long way and there are seldom complaints heard about nonreproducibility. Column packing is still considered by many to be a black art. Many laboratories have tried to study optimized packing conditions, but as particle sizes and particle chemistry change and column diameters become smaller, column performance has not been a linear transition. To realize equivalent performance with conventional analytical, narrow-bore, capillary, and nano columns, a more systematic study on column packing requirements will be needed.
Approaches to increase and predict chromatographic resolution with improved stationary phases that show better control of selectivity for critical separations will be needed in the future. Small changes in selectivity provide the biggest changes in overall resolution - much bigger than particle size effects alone, which affect only column efficiency. Decreases in particle size only give moderate increases in resolution (that is, R ≈ N1/2) . Most of the work in the past 25 years has been focused on improving efficiency, with many stationary phases based on commercially available silane reagents.
Although column lifetimes, even at higher pH values, are much longer nowadays than in yesteryear, many users (especially in the pharmaceutical environment) consider columns expendable. When dealing with precious, high-activity, high-value pharmaceuticals, compound purity and accuracy of analysis are of the utmost importance and a column that has been used for thousands of injections may have some degree of contamination that may affect retention and peak shape as well as compound purity, which is not worth risking in quantitative analysis. Some laboratories actually take perfectly good columns out of service that have reached 1000 analytical injections. Similar procedures are used for preparative columns that cost much more than analytical columns because of the increased amount of packing.
Supercritical Fluid Chromatography Columns: With its orthogonal separation power, supercritical fluid chromatography (SFC) has made a comeback in the rapid analysis of small pharmaceutical compounds. Initially, SFC made its contributions in the preparative arena for chiral drugs, but now it has been applied to more general small molecule applications. For some separations, SFC can be superior to HPLC and UHPLC, especially in the speed of analysis. The phases used for SFC (for example, ethyl pyridines, pyridyl amide, and DEAP) are different to those used for LC, so additional polar phases are required to exploit this technology. Further systematic studies on new phases by SFC column suppliers would add to the knowledge of reliably selecting the best stationary phase for a given separation (16).
Columns for Two-Dimensional LC: In the research community, two-dimensional (2D) LC has been gaining momentum when extremely difficult separations are encountered or when every compound in a complex sample needs to be separated (LC×LC). Here, truly orthogonal stationary phases are desired; so phases that are specifically designed for multidimensional separations could be on the horizon. Fast columns in the second dimension will be in popular demand and specialty phases based on SPP or monolithic technology may be needed to fill the gap. The 2D technique has not been accepted yet for routine pharmaceutical assays, but the day may come when more complete characterizations required by regulatory bodies may require this degree of separation power. Major instrument companies are already assembling multidimensional instruments to respond to this potential marketplace.
Stationary-Phase Chemistries: Reversed-phase chromatography has dominated HPLC column usage for the past four decades. Undoubtedly there are enough reversed-phase chromatography columns around to meet the needs of the entire chromatography community. Yet each year, tens of new reversed-phase chromatography columns are introduced because the market seems to be big enough to absorb some of these columns. Other modes do not get as much attention, but hydrophilic interaction chromatography (HILIC) has been growing from year to year. Many chromatographers continue to use bare silica for HILIC, even though there are a number of other HILIC phases available that provide unique selectivities. The mechanism for HILIC separations has become better understood. However, only a few new types of HILIC stationary phases have recently become commercially available to take advantage of this increased understanding. Since the HILIC mode in becoming more popular for polar analytes, unretained or slightly retained by reversed-phase chromatography, the market for HILIC columns has been growing. Likely, more specialized HILIC phases will be forthcoming. As more HILIC phases are introduced, a systematic study on how to choose the best HILIC column for the job at hand would be a welcome addition to the LC community.
The ion exchange-ion chromatography columns area has become a polymer only market. At Pittcon over the last several years, new silica-based ion-exchange columns have become almost nonexistent. The silica-based columns cannot stand up to the rigorous conditions used by ion-exchange separations, such as high ionic strength mobile phases, high pH, and high temperatures. Only a single ion-exchange polymeric monolith has been introduced in the last 5 years. It is anticipated that as monoliths become more established, ion-exchange chromatography could benefit from low-pressure-drop, high-efficiency monolith columns.
In the size-exclusion chromatography (SEC) area, particle sizes have seen a decrease giving rise to higher speed separations, but SEC is a technique where a sufficiently large stationary-phase volume is required to provide a large molecule weight operating range so making smaller internal diameter columns is probably not the answer. Column packings with noninteractive surface chemistries are always in demand, and in the organic SEC area (gel permeation chromatography), high-temperature-stable columns that stand up to somewhat exotic mobile phases are needed. Phases and column hardware for very high molecular weight polymers are required, mainly to reduce the high elongation strain rates that give rise to shear degradation. Very large pore packings with narrow particle size distributions for these type of polymers are usually very fragile, and it would be a real contribution to this field to finally solve the fragility problem.
Conclusion
It has been my pleasure for 33 years to write about columns and sample preparation for LC and LCGC. I hope that the magazine continues to thrive and provide useful and practical information on all aspects of chromatography to you, its loyal readers.
References
- R.E. Majors, LCGC North Am.25(1), 31-39 (2012).
- Extrapolated from Global Assessment Report, 2012-2016, Strategic Directions International (SDI), October, 2012.
- R.E. Majors, LCGC North Am.33(11), 818-840 (2015).
- R.E. Majors, LCGC North Am.27(4), 196-207 (2014).
- W. Barber, T.-C. Wei, W. Chen, A. Mack, J. Liu, M. Dittman, and X. Wang, “Next Generation Superficially Porous Particle Technology: Highly Ordered Pore Structure Formed by Pseudomorphic Transformation,” presented at HPLC 2015, Geneva, Switzerland, 2015.
- J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.W. Chu, D.H. Olson, and E.W. Sheppard, J. Am. Chem. Soc.114, 10834-10843 (1992).
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, and G.D. Stucky, Science279, 548-552 (1998).
- J.D. Glennon, University College Cork, US Patent US20110226990A1, 22 Sept. 2011.
- B.W. Muriithi and K.D. Wyndham, Waters, US Patent WO2012/018596A2, 9 Feb. 2012.
- T.-C. Wei, W. Chen, and W.E. Barber, Agilent Technologies, US Patent US8685283B2, 29 Aug. 2008.
- R.A. Henry, LCGC North Am.32(s4), 12-19 (2014).
- G. Desmet, D. Clicq, D.T.-T. Nguyen, D. Guillarme, S. Rudaz, J.-L. Veuthey, N. Vervoort, G. Torok, D. Cabooter, and P. Gzil, Anal. Chem.78, 2150-2162 (2006).
- G. Desmet, K. Broeckhoven, and D. Cabooter, personal communication, November, 2015.
- K. Broeckhoven and G. Desmet, Trends in Anal. Chem.63, 65-75 (2014).
- D. Cabooter, K. Broeckhoven, R. Sterken, A. Vanmessen, I. Vandendael, K. Nakanishi, S. Deridder, and G. Desmet, J. Chromatogr. A1325, 72-82 (2014).
- R. McClain and M. Przybyciel, LCGC North Am.29(10), 894-906 (2011).
Ronald E. Majors is the editor of “Column Watch,” an analytical consultant, and a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column should go to: “Column Watch”, LCGC Europe, Hinderton Point, Lloyd Drive, Ellesmere Port, CH65 9HQ, UK, or e-mail the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






