Frontiers of Sampling: Design of High Surface Area Thin-Film Samplers for On-site Environmental Analysis
LCGC North America
In on-site environmental applications, representative sampling and proper replication are essential. For these reasons, recent work in thin-film solidphase microextraction has focused on the development of unique holders and customized samplers that are tailored for distinct sampling environments. Here, we explore the latest developments.
In on-site environmental applications, representative sampling and proper replication are essential to properly characterize a given site. For these reasons, recent work in thin-film solid-phase microextraction (TF-SPME), an extension of fiber-based SPME, has focused on the development of unique holders and customized samplers that have been tailored for distinct sampling environments. Here, we explore the latest developments and designs of high-surface-area thin-film samplers for on-site environmental analysis using TF-SPME.
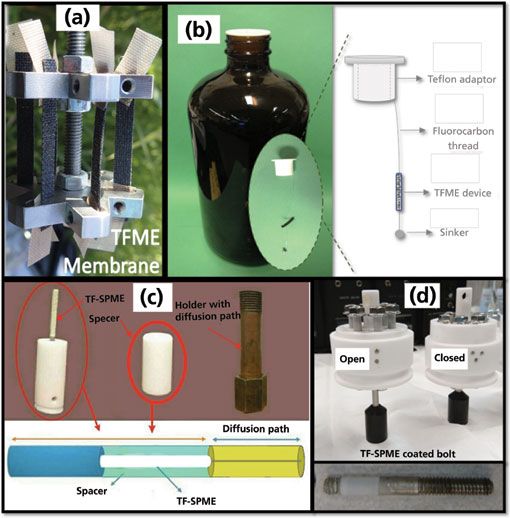
Figure 1: Compilation of various thin-film solid phase microextraction (TF-SPME) sampler formats including: (a) carbon mesh supported TF-SPME membrane on six-replicate drill sampler (7), (b) TF-SPME in-bottle grab sampler (6), (c) TF-SPME time weighted average sampler with hydrophilic lipophilic balance-particle-polyacrylonitrile (HLB-PAN) coated thin film blade (8), (d) TF-SPME coated bolt sampler in sampling (open) and storage (closed) position with HLB–PAN coated bolts (10).
We all know the saying, "Garbage in equals garbage out." With the proliferation of ultrahigh-pressure liquid chromatography (LC) systems and ever more sensitive mass spectrometry detectors, this adage that is more commonly thrown to the wayside in favor of simpler sample introduction approaches such as dilute-and-shoot. It can be easy to forget that where sampling and sample preparation precede instrumental analysis, any errors experienced during these crucial steps will affect the results, possibly leading an analyst to draw a false conclusion regarding his or her work. This holds especially true for on-site environmental applications, where representative sampling and proper replication are essential to satisfactorily characterize a given site (1,2). For these reasons, recent work in thin-film solid phase microextraction (TF-SPME), an extension of fiber based SPME, has focused on the development of unique holders and customized samplers that have been tailored for distinct sampling environments (3,4). These include more rigid carbon fabric supported membranes to give physical stability for high river flows and agitation (5), a six-replicate TF-SPME membrane holder that attaches to a specialized sampling case or standard power drill (6,7), in-bottle TF-SPME samplers for surface water grab sampling (6), broad spectrum sorbents for the simultaneous extraction of polar and nonpolar analytes (7), time-weighted average (TWA) membrane holders for long duration TWA river sampling (8), and even highly durable thin-film-coated bolts for the sampling of extreme environments (9,10). These devices are all shown in Figure 1. Much like fiber-based SPME, TF-SPME employs the fundamentals of thermodynamic equilibrium to drive the passive diffusion of small organic molecules, defined by the diffusion coefficient (Ds), across a static boundary layer of thickness (δ) that separates the free flowing sample matrix of analyte concentration (Cs) from the open sorbent bed. However, unlike an SPME fiber, thin-film devices are designed to have a total surface area (A) that is much larger than the sorbent thickness, resulting in extraction rates (dn/dt) that are orders of magnitude faster (11,12). This relationship is described mathematically in equation 1 below. Increased extraction kinetics are important to maximize the sensitivity of on-site environmental sampling approaches where one may be constrained to only a few minutes to collect each sample.
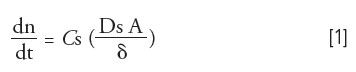
Appropriately, it was shown experimentally that when short, 15-min pre-equilibrium extractions were performed from water that had been spiked with various pesticides, a 40 mm × 4.8 mm (L × W) carbon fabric supported divinylbenzene/polydimethylsiloxane (DVB/PDMS) TF-SPME membrane could extract 21.2, 19.8, 18.5, 18,4, 26.8, and 23.7 times the amount of 2,4 dichlorophenol, 2,4,6 trichlorophenol, phorate D10, fonofos, chloropyrifos, and parathion than a comparable 65 µm DVB/PDMS SPME fiber respectively (5).This result was inline with what was expected fundamentally (equation 1), as the aforementioned TF-SPME membranes had a surface area that was approximately 25 times greater than that of the corresponding SPME fiber. Moreover, when directly compared to standardized liquid–liquid extraction, adopting a slightly modified US-EPA 8270 methodology, these sensitive membranes were shown to give MLOD values that were anywhere from 4 to 200 times lower than those achievable using LLE at a Standards Council of Canada accredited laboratory, while maintaining a similar level of accuracy in a double-blind split comparison (13). In fact, this benchtop TF-SPME methodology also required far less sample with all analytes being extracted simultaneously by directly immersing the membrane on a cotter pin into 30 mL of spiked surface water at a pH of 2.5, with 10% NaCl and a stir rate of 900 rpm. This was in sharp contrast to the LLE approach, which required three sequential 50 mL dichloromethane extractions from 800 mL of spiked surface water at acidic, neutral, and basic conditions. With these results in mind, similarly designed membrane holders, shown in Figure 2, were included with the recent commercial release (Gerstel US) of the carbon mesh supported TF-SPME membrane (14).
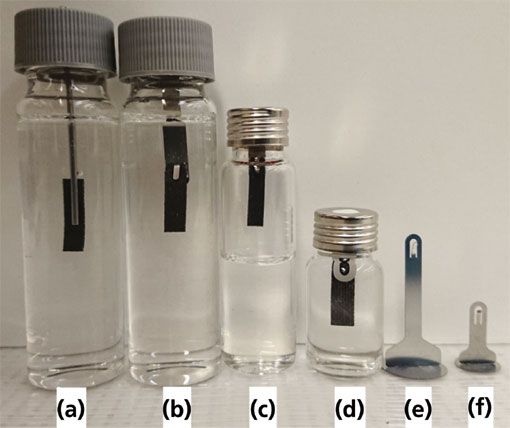
Figure 2: In-vial thin-film solid phase microextraction (TF-SPME) membrane holders including: (a) prototype cotter pin TF-SPME holder pushed through septa used for inter-laboratory study (13), (b) comparable commercial membrane holder for direct immersion extraction from 40 mL vial, (c) TF-SPME holder set-up for 10 mL headspace extraction (14), (d) TF-SPME holder set-up for 10 mL direct immersion extractions, (e) TF-SPME holder for 40 mL vial, (f) TF-SPME holder for 10 and 20 mL vials.
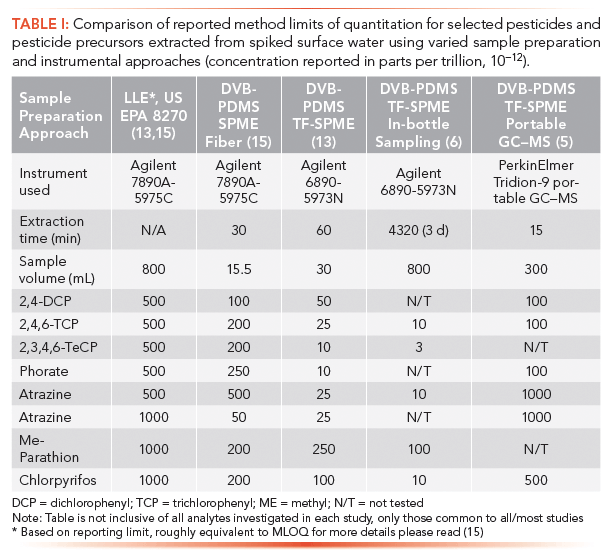
To further improve the applicability of TF-SPME related methods, subsequent research focussed on the development of more novel TF-SPME holders to allow for unique sampling opportunities. In a recently published study, Piri-Moghadam and associates compared an in-bottle TF-SPME methodology to on-site sampling approaches with and without hand portable gas chromatography-mass spectrometry (GC–MS) instrumentation (6). This in-bottle approach was devised in a manner such that the sampling process was not a major departure from conventional grab sampling. When applying the bottle sampling approach, a technician simply needs to place the TF-SPME–equipped PTFE adapter, shown in Figure 1b, and spike an appropriate internal standard into the 800 mL glass bottle when collecting a surface water sample. Following sampling, these bottles were then allowed to sit on an orbital shaker for three days pending analysis by use of a Gerstel thermal desorption unit and cooling injection system (TDU-CIS4) equipped Agilent 6890-5973N GC–MS instrument. As shown in Table I, this in-bottle approach gave the lowest limits of quantitation observed in the study with MLOQ values typically ranging between 1–10 ppt for most analytes. A more novel approach also explored the use of the custom six-replicate TF-SPME membrane holder shown in Figure 1a to perform on-site extraction directly from river water. These membranes were then either analyzed directly on-site using a hand-portable Tridion-9 GC–MS instrument, or transported back to the laboratory for desorption and analysis using the aforementioned TDU-CIS4 equipped Agilent 6890-5973n benchtop GC–MS instrument (6). Although the targeted, banned pesticides could not be detected at appreciable levels from Ontario Rivers during the study, a comparison of the MLOQ values for a selection of these compounds indicated that the entirely on-site TF-SPME methodology could provide quantitative limits similar to those of conventional benchtop LLE and SPME. The comparison of these methods and associated MLODs are listed in Table I.
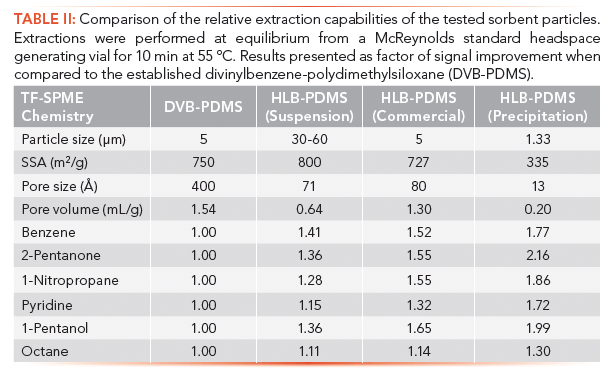
Following the optimization and validation of these carbon mesh supported TF-SPME membranes and their various specialized holders, research efforts were shifted toward the development of more sensitive sorbents, particularly those with a broader affinity for polar analytes. Appropriately, hydrophilic lipophilic balance (HLB) particles, based on a co-polymerization of N-vinylpyrrolidone onto a divinylbenzene backbone, made for a logical choice to compare to the now validated DVB/PDMS carbon mesh supported membranes (7). In this study, three types of HLB particles, differing in their particle and pore size, were compared to the established DVB-based thin-film membranes. From the three HLB particles tested, one came from a commercial source, while the other two were synthesized in-house using either a precipitation, or suspension polymerization methodology. All particles were suspended in PDMS, spread onto the carbon fabric support and cut to 20 mm × 4.8 mm to fit the TDU-CIS4 system as before.
It was observed that the commercial HLB particles, those prepared by suspension polymerization, and the DVB particles had similar specific surface areas (727–800 m2/g), but demonstrated greatly different extraction capabilities for the targeted McReynolds mixture. To better understand this result, complete characterization of the particles using Fourier-transform infrared spectroscopy, elemental analysis and Brunauer-Emmett-Teller analysis were performed, indicating that despite having a similar proportion of the N-vinylpyrrolidone moiety, the diameter of the pores and total pore volume for the sorbents were substantially different. HLB particles synthesized by suspension polymerization and obtained from commercial source had pore sizes of 80 and 71 angstroms (Å) and pore volumes of 1.30 and 0.64 mL/g, respectively, making them generally mesoporous. In the meantime, the DVB particles had the largest pores, with a diameter of 400 Å and volume 1.54 mL/g. Finally the smallest pores were observed in those particles prepared by precipitation polymerization with an average pore diameter of 13 Å and volume of 0.20 mL/g. Surprisingly, despite having the smallest pore volume, the HLB particles synthesized by precipitation polymerization gave the best results, extracting nearly double the amount of the polar standards than the DVB/PDMS membranes (Table II). This result was attributed to the fact that the precipitation polymerized HLB particles had a generally microporous structure stemming from the use of acetonitrile as a porogen. Moreover, the smaller 1.33 µm particle diameter could have also played an important role considering the spread distribution and presence of a higher number of particles on the membranes. Unsurprisingly, as shown in Table II, it was found that all of the HLB sorbents tested did in fact outperform the established DVB particles for all of the McReynolds standards tested. This increase in the affinity for semipolar analytes can be attributed to the presence of the N-vinylpyrrolidone group. These groups provide sites for hydrogen bonding, and allow the hydrophilic functionalities to interact more strongly with the sorbent. Although not as pronounced, it was somewhat surprising to also see slightly increased performance offered by the HLB particles for the octane standard added to the McReynolds mixture. With a LogP value of 5.01, one would expect octane to be primarily extracted by the nonpolar interactions offered by the divinylbenzene. It is also important to note that instrumental runs were fully randomized during the experiment, ruling out the possibility of detector drift during experimentation. Speculatively, it is possible that this result could also be explained by the much smaller pore diameter present in all of the HLB materials tested.
Interestingly, it was also found that the physical characteristics of the carbon mesh support allowed for an innovative calibration approach for converting raw instrument signal to tangible amounts such as nanograms. This calibration approach, called on-membrane liquid injection, allowed for liquid standard to be introduced to the GC–MS instrument, without having to remove the TDU-CIS4 system or install a liquid injection syringe onto the autosampler. Instead, it was possible to manually spike a few microliters of a methanolic McReynolds standard directly onto the edge of the TF-SPME membrane, that then wicked the solution into the carbon mesh support. Once analyzed, it was found that an exceptional correlation to response (R2 ≥ 0.996) for all analytes could be achieved while most of the volatile methanol solvent was purged from the CIS4 during analyte reconstitution, never reaching the GC column. Moreover, it was also found that once extracted onto the TF-SPME membranes and stored within the Gerstel thermal desorption tubes on the autosampler rack, all of the McReynolds standards were stable on-membrane for up to 24 h. In fact, only the photosensitive pyridine was shown to deplete when this test was further lengthened to 120 h.
Having shown favorable results for polar VOC analysis, these HLB/PDMS membranes were then applied for an untargeted, on-site determination of chlorination byproducts from a private hot tub. For this proof of concept application the HLB/PDMS thin films prepared using the precipitation polymerized HLB were cut to 40 mm × 4.8 mm (L × W) for analysis on the Tridion-9 portable GC–MS system. As shown in Figure 3, sampling was accomplished by placing four membranes onto the six-replicate TF-SPME holder, which was connected to a sampling case (PAS Technologies GmbH), allowing for extractions to be performed at 2000 rpm for 10 min from the 39.5 °C, pH 7.2–7.4 hot-tub water. Immediately after sampling, the membranes were removed from the drill, and dabbed dry with a Kimwipe before being placed in the 3.5-in. thermal desorption tubes. These tubes were then inserted into the SPS-3 module and desorbed at 250 oC using 35 mL/.min of helium to transfer the desorbed analytes to a 19 gauge Tenax/Carboxen needle trap device (NTD). This NTD could then be placed directly into the Tridion-9 portable GC–MS instrument for desorption and analysis of the compounds transferred from the TF-SPME membranes. In total, six different chlorination by products were identified from the hot tub water. This identification was performed by matching with the NIST 2011 mass spectral database, followed by preliminary verification by use of a standard n-alkanes linear retention index plot. These compounds included chloroform, bromodichloromethane, dichloroacetonitrile, chlorobenzene, benzonitrile, and benzyl chloride, all of which were known disinfectant by-products.

Figure 3: On-site hot tub sampling set-up showing: (a) the flexible cable drive attachment of the thin-film solid phase microextraction (TF-SPME) sampling head for direct analysis of chlorination byproducts from a hot tub, (b) the SPS-3 portable desorption module undergoing TF-SPME desorption, and, (c) the Tridion-9 portable GC–MS. Sampling was performed at 2000 rpm and the hot tub was measured to be 39.5 °C with a pH of 7.2–7.4.
Finally, two of these compounds were quantified using a matrix-matched external calibration curve at five concentration levels (n = 3), ranging from 25 to 250 µg/L for chloroform, and 125 to 1000 µg/L for dichloroacetonitrile. Calibration was performed in 3 L of matrix-matched water which yielded R2 values over 0.99, indicating very good correlation to response for the TF-SPME equipped portable GC–MS instrument. The chloroform and dichloroacetonitrile were found to be present at a concentration of 270 µg/L and 79 µg/L, having RSDs of 13% and 5%, respectively (n = 4). It is worth noting that the World Health Organization (WHO) considers chloroform levels up to 300 µg/L and dichloroacetonitrile levels approaching 20 µg/L to be safe for bathing and drinking (16).
Of course, TF-SPME devices are not exclusive to GC-based applications. There has been a great deal of work performed on HPLC-amenable thin-film blades, as shown in the TWA holder of Figure 1c. These blades are generally manufactured using a more porous and permeable polymeric binder, such as polyacrylonitrile (PAN), to facilitate solvent desorption of the suspended sorbent particles. In this study, performed by Ahmadi and associates (8), the aforementioned TWA-TF-SPME-HPLC amenable sampler was shown as an environmentally friendly means to extract various biocides and ultraviolet (UV) blocking agents of mixed polarity from aqueous environments. These thin-film blades were prepared with either HLB/PAN or C18/PAN. As shown in Figure 1c, the TWA sampler was constructed such that the thin film blade was mounted onto a chemically inert polytetrafluoroethylene (PTFE) holder, and then covered by another PTFE spacer tube to reduce the dead-volume within the device. This assembly was then inserted into an anti-biofouling copper tube with a predrilled diffusion path with a length (Z) of 10 mm, and cross sectional area (A) of 0.49 mm2. When combined with the known static diffusion coefficients (D) of a given analyte, the sampling time (t) and the total amount extracted over the sampling period (n) these values can be combined to create equation 2 and calculate the time weighted average concentration (Cavg). After validating the accuracy of the TWA assembly and that analyte uptake remained linear for 70 days using an in-lab simulated river system, the samplers were deployed on wastewater affected portions of the Grand River, Ontario, for a period of 90 d. Although not all of the targeted benzophenone compounds were above the MLOQs of the TWA methodology, the time weighted average concentrations of Benzophenone-4 and 2-phenylbenzimidazole-5-sulphonic acid (PBSA) were determined to be 5.4 parts per billion (ppb) and 4.0 ppb over the 90-d sampling period. This was found to be in good agreement with corresponding grab sampling data giving respective concentrations of 4.5 and 2.3 ppb in June when the TWA devices were deployed and, at levels of 6.6 ppb and 4.9 ppb when the TWA samplers were collected in August.

Moving forward, the principle of thin-film devices does not need to be restricted to flat planar shapes, such as the abovementioned fabric supported membranes and thin-film blades. As facilitated by solvent desorption, adsorbent thin film coatings can be applied to solid supports of varying size and shape. Fittingly, in a very recent and innovative study performed by the Pawliszyn Research Group, a new design of TF-SPME was explored by applying a thin, 25 µm, coating onto 6 mm diameter cylindrical bolts. Shown in Figure 1d, these bolts were then used to develop a robust, sealable TF-SPME sampler for the on-site sampling and extraction of a wide range of untargeted pollutants in environmental water samples. This design was tailored to allow for the extraction of a broad spectrum of mixed polarity compounds from extreme environmental waters while offering a means to stabilize the extracted compounds on the sorbent coatings for extended periods in ambient conditions. In order to properly test these devices, a real world proof of concept was performed for the analysis of untargeted pollutants in water samples from five different rivers in China and Canada (10).
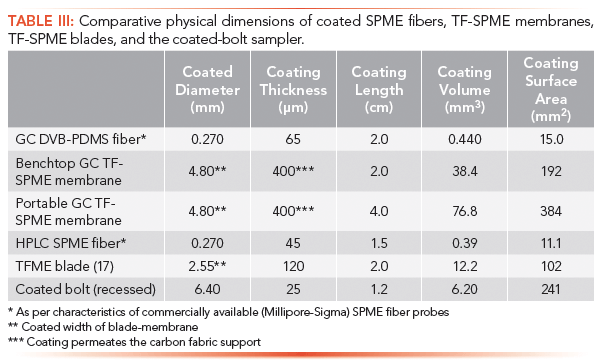
Coated bolts were prepared by using dip coating to deposit an HLB/PAN sorbent within a 1.2 cm long recession on the stainless steel bolts. As can be seen in Table I, among all of the currently discussed TF-SPME morphologies, the coated bolt layout provides a considerably large surface area, larger than that of any of the other HPLC amenable devices. This large 241 mm2 surface area is needed to achieve adequate sensitivity during the relatively short sampling times expected for on-site sampling. With these factors in mind, one could expect a signal improvement by a factor of 22 times over a comparable HLB/PAN SPME fiber when short pre-equilibrium extractions are performed.
To confirm that the sampler was able to stabilize extracted compounds for long periods of time, the effects of storage time and temperature were evaluated. This was accomplished by deploying three coated bolt samplers at the outflow pipe of an undisclosed wastewater treatment facility for approximately 1 h via kayak (Figure 4). Upon retrieving the samplers, they were sealed and transported back to the lab where four of the 18 bolts were removed, and immediately desorbed by use of solvent for analysis on a high-resolution HPLC-orbital ion trap-MS instrument. The remaining 14 bolts were then stored in one of three ways before desorption and analysis including: 3 d at room temperature, 12 d at room temperature, and 12 d within a -80 oC freezer. The results of this test showed no significant differences in the quantity and quality of the extracted chemicals following any of the storage conditions, thus confirming the device's suitability for use at sampling sites that are far away from the laboratory facilities (10).
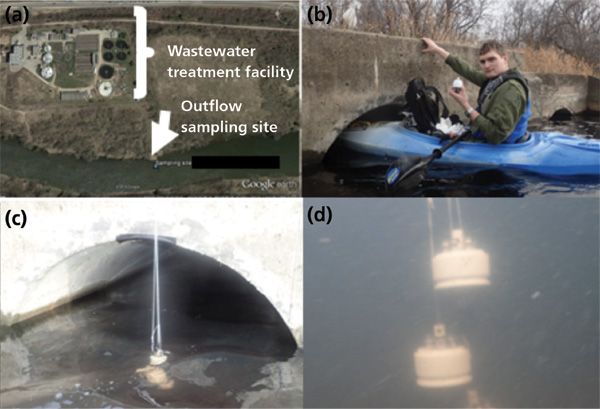
Figure 4: Coated bolt thin-film solid phase microextraction (TF-SPME) sampler deployment via kayak at an undisclosed wastewater treatment facility in Ontario, Canada.
A full application of these samplers was performed by sampling four different rivers in China, including the Pearl River in the city of Guangzhou, the Yangtze River in Wuhan, a tributary of the Yangtze River in Shanghai, and the Xinghai River in Dalian (10). After returning to Canada, these samplers were desorbed and analyzed using high-resolution HPLC-orbital ion trap-MS, followed by multivariate statistical processing, to tentatively select significant features from each of the sampling sites. The Toxin and Toxin-Target Database was used as a reference for toxins and environmental contaminants. Eventually, more than 80 tentative compounds with widely varying hydrophobicity's from -2.43 < logP < 11.9 were determined. These compounds included a variety of drugs, metabolites, miscellaneous toxins, and pesticides, which were extracted by the sampler from these different rivers. It is important to note, however, that although relative HPLC retention times and adduct annotation were used to further improve the reliability of the results, these identifications were still tentative in nature. Regardless, the logP values of these tentatively identified compounds indicate that these devices were in fact capable of extracting and stabilizing a wide variety of mixed polarity compounds from real environmental samples.
Conclusions
Solid-phase microextraction can come in many shapes and forms, with recent research indicating the high surface area thin-film morphologies are likely the ideal choice in performing environmental analysis, whether in the laboratory or on-site. Moreover, the design of a given TF-SPME sampler and holder can greatly enhance usability, sensitivity, and reliability, if these are properly designed for a given application, whether this be the six-replicate TF-SPME holder which allows for on-site agitation, broad polarity HLB-based sorbent for GC and LC amenable thin films, and even a sealable coated bolt sampler that can stabilize extracts for weeks when refrigeration and dry ice are not available. These deployments show just how important sampler design and sample preparation are for improving the sensitivity and reliability of results generated from our ever improving analytical instrumentation.
References
(1) K.N. Engler and A.T. Lemley, Environ. Toxicol. Chem. 32(9), 1962–1968 (2013).
(2) N. Strittmatter, R.-A. Düring, and Z. Takáts, Analyst 137(17), 4037 (2012).
(3) I. Bruheim, X. Liu, and J. Pawliszyn, Anal. Chem. 75(4), 1002–1010 (2003).
(4) F. Riazi Kermani and J. Pawliszyn, Anal. Chem. 84(21), 8990–8995 (2012).
(5) J.J. Grandy, E. Boyaci, and J. Pawliszyn, Anal. Chem.88(3) (2016).
(6) H. Piri-Moghadam, E. Gionfriddo, J.J. Grandy, M.N. Alam, and J. Pawliszyn, J. Chromatogr. A 1579, 20–30 (2018).
(7) J.J. Grandy, V. Singh, M. Lashgari, M. Gauthier, and J. Pawliszyn, Anal. Chem. 90(23), 14072–14080 (2018).
(8) F. Ahmadi, C. Sparham, E. Boyaci, and J. Pawliszyn, Environ. Sci. Technol. 51(7), 3929–3937 (2017).
(9) N. Reyes-Garcés et al., Anal. Chem. 90(1), (2018).
(10) J. J. Grandy, M. Lashgari, H. Vander Heide, J. Poole, and J. Pawliszyn, Environ. Pollut. 252, 825–834 (2019).
(11) B. Bojko et al., Anal. Chim. Acta 750, 132–151 (2012).
(12) N. Reyes-Garcés et al., Anal. Chem. 90(1), 302–360 (2018).
(13) H. Piri-Moghadam et al., Anal. Chim. Acta 964(July 2016), 74–84 (2017).
(14) J.R. Stuff, J. A. Whitecavage, J.J. Grandy, and J. Pawliszyn, Gerstel application Note (200), 1–10 (2018).
(15) A. Rodriguez-Lafuente, H. Piri-Moghadam, H.L. Lord, T. Obal, and J. Pawliszyn, Water Qual. Res. J. Canada 51(4), 331–343 (2016).
(16) H.G. Gorchev and G. Ozolins, WHO Chron. 38(3), 104–108 (2011).
(17) E. Cudjoe, D. Vuckovic, D. Hein, and J. Pawliszyn, Anal. Chem. 81(11), 4226–4232 (2009).
Jonathan Grandy, Maryam Lashgari, Varoon Singh, and Janusz Pawliszyn are with the Department of Chemistry at the University of Waterloo, in Waterloo, Ontario, Canada. Direct correspondence to: janusz@uwaterloo.ca

Identification of Microplastics in Environmental Monitoring Using Pyrolysis–GC–MS Analysis
April 7th 2021Pyrolysis–gas chromatography–mass spectrometry has advantages for the analysis of environmental microplastic samples compared to other leading analytical methods, including spectroscopic techniques.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






