Extending the Detection Limits for the Analysis of Organotin Contaminants Using Soft Ionization
The Column
The inherent sensitivity and selectivity of time-of-flight mass spectrometry (TOF-MS) can be augmented by soft electron ionization (EI) to provide ultratrace-level quantitation of organotins in complex environmental extracts. These organotin species are a focus of current concern as environmental contaminants, but analysis using conventional 70 eV ionization energies is made difficult by their propensity to undergo extensive fragmentation. The use of soft EI helps to solve this problem by producing simplified spectra with enhanced diagnostic ions.
Photo Credit: Dennis Gerbeckx/Getty Images

Laura McGregor, Steve Smith, and David Barden, Markes International, Llantrisant, Wales, UK
The inherent sensitivity and selectivity of time-of-flight mass spectrometry (TOF-MS) can be augmented by soft electron ionization (EI) to provide ultratrace-level quantitation of organotins in complex environmental extracts. These organotin species are a focus of current concern as environmental contaminants, but analysis using conventional 70 eV ionization energies is made difficult by their propensity to undergo extensive fragmentation. The use of soft EI helps to solve this problem by producing simplified spectra with enhanced diagnostic ions.
Organotins (stannanes) are anthropogenic chemicals that are attracting attention from environmental analysts because of their high toxicity and ability to interfere with the endocrine system, along with their persistence in the environment. Use of organotins in anti-fouling paints first caused concern in the 1970s as a result of a decline in populations of marine molluscs. The use of such paints has now been restricted or banned by many countries, but other sources remain, such as PVC products, disinfectants, and agricultural pesticides.1
The current annual average of organotins in water, as stated by the EU Water Framework Directive, is just 0.2 ng/L, with a maximum allowable concentration of 1.5 ng/L.2 This means that highly sensitive detection methods are required, with environmental analysts constantly seeking improvements to instrumentation and technological advances that could lower reporting limits.
Time-of-flight mass spectrometry (TOF–MS) offers significant advantages in such scenarios, with instruments using direct extraction (rather than the inherently less efficient orthogonal extraction) providing a distinct sensitivity advantage. However, certain analytes remain challenging even with such technologies because of their propensity to fragment extensively at conventional (70 eV) ionization energy.
This problem can now be addressed by the use of a soft electron ionization (EI) technique that offers the advantages of soft ionization for gas chromatography coupled to MS
(GC–MS), without any of its historic disadvantages - such as hardware changes and additional reagent gases. The technique uses an ion source design in the TOF instrument that allows the ionization energy to be varied on a sliding scale from conventional 70 eV to lower energies.3 The physical properties of most small molecules means that relatively small differences in ionization energies between 10 eV and 20 eV can have significant differences in the fragmentation pattern. Typically, however, an ionization energy of about 12–14 eV retains an useful degree of fragmentation while avoiding a loss in sensitivity relating to the unavoidable drop-off in ionization efficiency as the ionization potential is approached.
This technique has already been applied to analytes ranging from petrochemical hydrocarbons4 to emerging environmental contaminants.5 This article explains how this soft ionization method can be applied to organotin compounds. These are particularly challenging because of the requirement for high sensitivity to meet the demands of the low detection limits, in conjunction with their extensive fragmentation at 70 eV making speciation difficult. Using soft ionization provides enhanced “diagnostic” ions and reduced chemical noise, which leads to lower detection limits, as well as simplified spectra for more confident qualification. A further boost in sensitivity is demonstrated by reduced ionization of common background/carrier gases at low ionization energies, leading to minimal chemical noise and improved signal-to-noise ratios for compounds of interest.
Experimental
Samples: A selection of some of the most common organotins were analyzed in this work: dibutyl tin (Bu2SnH2), tributyl tin (Bu3SnH), monooctyl tin ([C8H17]SnH3), and tetrabutyl tin (Bu4Sn). Tri-substituted organotins such as tributyltin are used as pesticides,6 while mono- and di-substituted organotins are used as PVC stabilizers, as catalysts, and in glass coatings.7 The lower–substituted organotins can also be formed in the environment by metabolism and degradation of higher-substituted analogues.6 Environmental water samples were spiked with organotins (50 ng/L) and ethylated with sodium tetraethylborate prior to extraction to make the tin hydrides sufficiently volatile for analysis by GC–MS. A dilution series (ranging from 0.1–20 ng/L) was prepared from the stock solution.
GC: Carrier gas: Helium, constant flow at 0.9 mL/min; mode: splitless for 2.0 min (then 150 mL/min purge); temperature: 200 °C; septum purge: on, 3 mL/min; column: 20 m × 0.18 mm, 0.18-µm Rxi–5Silms (Restek); oven: 50 °C (2.5 min), 20 °C/min to 300 °C (1 min); total run time: 16 min.
TOF-MS: Instrument: BenchTOF-Select (Markes International); filament voltage: 1.8 V; ion source: 250 °C; transfer line: 280 °C; mass range: m/z 40–500; data rate: 4 Hz.
Software: TOF-DS software for BenchTOF (Markes International) was used for full instrument control and data analysis.
Results and Discussion
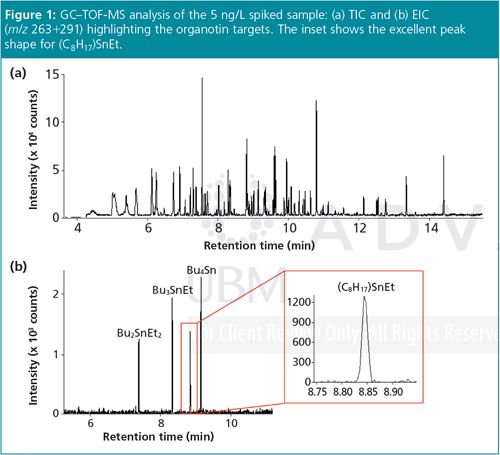
Figure 1 shows the GC–TOF-MS analysis of the 5 ng/L spiked sample, with an extracted ion chromatogram (EIC) indicating the elution order and excellent peak shape and intensity for the organotin targets, even at this trace level (5 pg on–column).
Spectral Quality: The spectra obtained for each of the organotin target compounds at 70 eV and 14 eV are compared in Figure 2. The results show that softer (14 eV) ionization simplifies the spectra and increases the intensity of the higher m/z ions. This is important because these larger fragments are useful for determining compound structure.
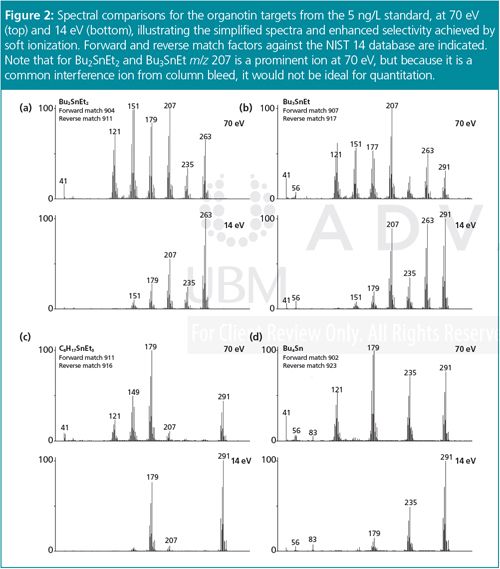
Furthermore, the preservation of a degree of fragmentation provides more information than other soft ionization techniques (such as chemical ionization) that may produce spectra containing solely the molecular ion. Library–searching can therefore be performed at low eV to add another level of confidence in compound identification.
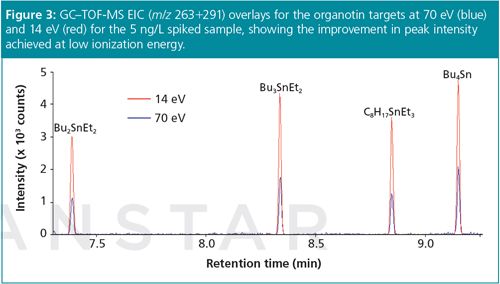
Figure 3 shows the EIC (m/z 263+291) overlays for 14 eV and 70 eV, illustrating the improved detection limits at low eV. Note the reduced baseline at 14 eV, as a result of reduced ionization of common background/carrier gases, further improving signal–to–noise ratios for the peaks of interest.
Linearity: The set of derivatized organotins was analyzed at six dilution levels by GC–TOF-MS at ionization energies of 70 eV and 14 eV. The resulting calibration curves (Figure 4) display excellent linearity at both ionization energies, with all R2 values over 0.997. Note in particular the steeper calibration curves at 14 eV, which result in increased analyte response factors and therefore lower quantitation limits.
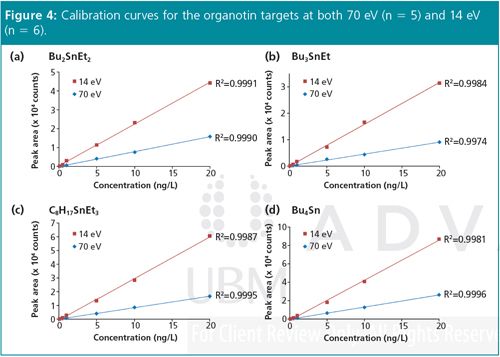
None of the organotins at the lowest dilution level (0.1 ng/L) could be reliably detected at 70 eV (and so this data point is not shown in Figure 4), but the enhanced sensitivity at 14 eV easily enabled detection at this level (Figure 5).
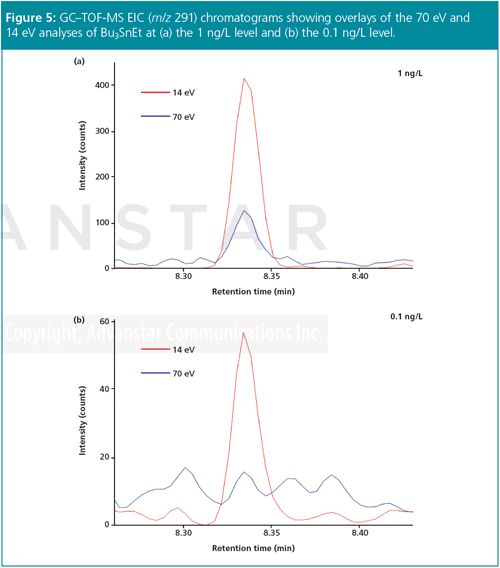
Conclusion
This article has demonstrated the performance of this GC–TOF-MS method for trace-level detection and quantitation of organotins in complex matrices.
The soft ionization technology used provided a level of sensitivity and selectivity not achievable with other methods, and spectra exhibiting enhanced diagnostic ions for all organotins at 14 eV. Excellent linearity was achieved (R2 > 0.997) for both conventional (70 eV) and soft (14 eV) ionization. Moreover, the enhancement in quantitation ions and reduced background at 14 eV enabled detection limits to be extended down to just 0.1 pg on-column for all organotins in this study.
References
- H.K. Okoro, O.S. Fatoki, F.A. Adekola, B.J. Ximba, R.G. Snyman, and B. Opeolu, in Reviews of Environmental Contamination and Toxicology, (vol. 213, ch. 2, 2011) pp. 27–54.
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water. See http://ec.europa.eu/environment/water/water-framework/index_en.html
- L. McGregor, N. Bukowski, and D. Barden, Current Trends in Mass Spectrometry, supplement to LCGC North America, LCGC Europe, and Spectroscopy, 12(1), 16–19 (2014).
- L. McGregor and D. Barden, Analyzing crude oil: Improving compound speciation, Hydrocarbon Engineering, July 2014, http://www.energyglobal.com/downstream/gas-processing/31072014/Crude-oil-markes-analysis/
- L. McGregor, A. Gravell, I. Allan, G. Mills, D. Barden, N. Bukowski, and S. Smith, The Analytical Scientist April 2015.
- S. Dobson and R. Cabridenc, Tributyltin compounds (Environmental Health Criteria 116), World Health Organization (1990).
- S. Dobson and P.D. Howe, Mono- and disubstituted methyltin, butyltin, and octyltin compounds (Concise International Chemical Assessment Document 73), World Health Organization (2006).
Laura McGregor received an M.Chem. in chemistry from the University of St Andrews, UK, followed by an M.Sc. in forensic science at the University of Strathclyde, UK. Her Ph.D. in environmental forensics, also at the University of Strathclyde, focused on the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC−TOF-MS. Laura joined Markes International in 2013 as a sales support specialist, and is now product marketing manager for Markes’ TOF–MS product portfolio.
Steve Smith studied in Bristol, UK, for both his B.Sc. and Ph.D., which he obtained in 2008 on innovative work profiling volatile organic compounds for disease diagnosis. Following post–doctoral positions at the University of the West of England and Bristol University, Steve joined Markes International as a senior applications specialist for thermal desorption and TOF–MS in 2011, where he now specializes in GC×GC−TOF-MS.
David Barden is a technical copywriter at Markes International, having joined the company in 2011. David studied natural sciences at the University of Cambridge, UK, and remained there for his Ph.D. in organic chemistry, which he received in 2003. A placement at the European Journals Department of Wiley-VCH, Weinheim, Germany, was then followed by seven years in journal publishing at the Royal Society of Chemistry, UK.
E-mail: enquiries@markes.com
Website: www.markes.com

Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.
Potential Obstacles in Chromatographic Analyses Distinguishing Marijuana from Hemp
April 28th 2025LCGC International's April series for National Cannabis Awareness Month concludes with a discussion with Walter B. Wilson from the National Institute of Standard and Technology’s (NIST’s) Chemical Sciences Division regarding recent research his team conducted investigating chromatographic interferences that can potentially inflate the levels of Δ9-THC in Cannabis sativa plant samples, and possible solutions to avoid this problem.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












