Exploring the Implementation of Compact Chromatographic Instrumentation in Common Analytical Workflows
In many industries, the concepts of “smaller,” “faster,” “easier to use,” and “cheaper” are key drivers when developing new technologies. The world of chemical separations is no different. Today, compact chromatographic instrumentation is being implemented in many workflows, as illustrated here through various examples.
The field of compact (or portable) chromatography has been a frequent topic of discussion in the pages of LCGC recently, with contributions from our group (1) and others in the field (2–4) over the past 16 months. The broad advantages of miniaturized chromatographic instrumentation include reduced benchtop footprint and design simplification in both system operation and software control. Portability, decreased costs for consumables, and a reduction in waste generation can also be achieved, depending on the specific system. Ideally, the systems will achieve performance that matches or exceeds conventional benchtop equipment, although this can be difficult to accomplish while maintaining a small size and low cost. Our approach to implementing these compact chromatographic instruments in our research program has been through the practical approach of identifying areas in which simplified analytical tools can fill a particular need currently underserved by standard measurement strategies.
James P. Grinias, Associate Professor of Chemistry, Rowan University

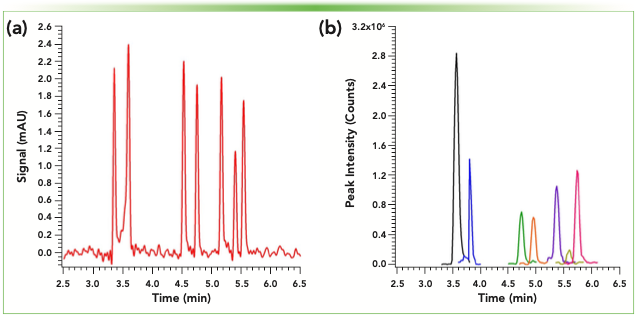
One of these areas is related to point-of-care (POC) monitoring in clinical settings. We are specifically interested in improving the capability of healthcare professionals to test for recent drug usage, as this can aid in selecting an appropriate course of treatment. In POC settings, this testing is most often achieved by using lateral flow immunoassays to screen urine samples for drugs of abuse (5). Although this strategy can typically identify the presence of specific drug classes, it often suffers from cross-reactivity within a given class, making it hard to identify which specific compounds may be in a patient’s system. For broad drug classes, such as benzodiazepines, having this additional information may require chromatographic-based testing at an external laboratory facility, which can delay results for several days. Identifying the presence of these compounds has become increasingly relevant in drug screening, as overdose deaths attributed to combinations of opioids and benzodiazepines increased tenfold from 1999 to 2017 (6). In Figure 1a, an early result in the development of a rapid, liquid chromatography (LC)-based assay for measuring various benzodiazepines using portable instrumentation is shown. Analyte concentrations for this sample were in the 5–10 ppm range, which is approximately 20- to 30-fold higher than the cutoff level concentration required for screening. Here, an on-capillary light-emitting diode-ultraviolet (LED-UV) detector was used, so the absorbance pathlength was limited to 0.150 mm, which is much shorter than a typical flow cell in a benchtop instrument. This value can be increased by using slightly larger i.d. columns coupled to the on-column detector, or integrating a low-volume, post-column flow cell, both of which are being investigated. Furthermore, sample preparation techniques such as solid-phase extraction can be used to preconcentrate samples and improve detection limits. More-sensitive detection techniques, such as mass spectrometry, can also be used, as shown in Figure 1b. Here, a compact single quadrupole MS system designed for low infusion flow rates can be easily coupled to the column outlet of the portable capillary LC instrument. Selected ion monitoring over targeted retention time windows allows for targeted detection of relevant compounds, along with significant improvements in signal-to-noise for the same sample concentrations. However, adding this second detector increases the size and complexity of the overall system, which may be a hurdle to implementation in certain POC environments. As we continue to develop methods for benzodiazepines and other drug classes using compact LC–UV and LC–MS instrumentation (7), we anticipate that the best techniques require a delicate balance between high performance, high throughput, decreased operation cost, small size, and ease of use for healthcare professionals.
FIGURE 1: (a) Capillary LC–UV, and (b) capillary LC–MS (selected ion chromatograms) showing separations of a benzodiazepine mixture with a 0.150 x 100 mm C18 column (1.7 μm fully porous particles) using Axcend Focus LC and Microsaic MiD-4500. Elution order (as well as concentrations and trace color): flurazepam (10 ppm, black), bromazepam (5 ppm, blue), nitrazepam (10 ppm, green), lorazepam (10 ppm, orange), flunitrazepam (5 ppm, purple), clobazam (5 ppm, gold), and diazepam (5 ppm, pink). 40 nL of sample was injected onto column. Mobile phase consisted of water and acetonitrile (with 0.1% formic acid) with a 25–75%B gradient over 7 min at a 1.5 μL/min flow rate.
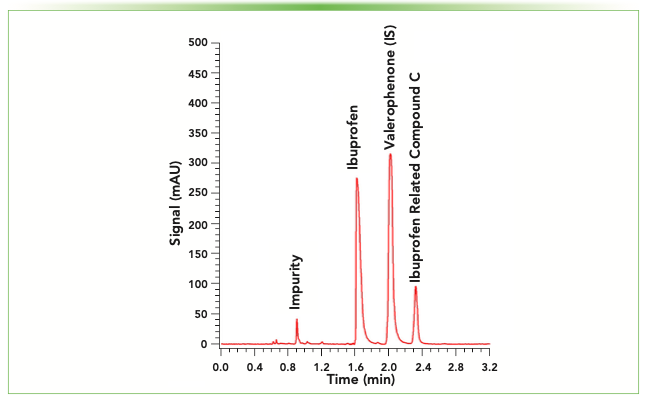
As our department houses a PhD in Pharmaceutical Chemistry program, our research group also has an interest in pharmaceutically relevant chromatography applications. Recent work has focused on the development of higher throughput approaches to monograph-based separations of over-the-counter (OTC) analgesic drugs (8,9). These techniques showed reductions in both method cycle time and sample waste generation compared to the monograph methods. Although this approach demonstrates the power of modern chromatographic technology, the implementation of these strategies in manufacturing workflows can be difficult due to the use of higher performing ultrahigh-performance liquid chromatography (UHPLC) instrumentation. The production facilities where these large-batch OTC compounds are generated typically rely on methods with 4.6 mm i.d. columns operated on standard HPLC instrumentation, as significant changes to existing methods sometimes requires extensive regulatory paperwork. In these instances, updated compact instrumentation can achieve similar performance to existing commercial instrumentation, but with reductions in overall size and cost. In Figure 2, a miniLC system was used for a combined drug product content and impurity assay for ibuprofen, using conditions based upon a published monograph method (10). As most published monograph methods for OTC compounds were designed for use with similar columns on instruments with comparable capabilities, many pharmacopeial separations could be easily translated to this lower-cost platform. We have only recently begun exploring the use of this platform, and we anticipate it may be useful in a number of other LC applications in our laboratory, including those related to cannabinoid potency testing as part of our department’s new Cannabinoid Chemistry certificate program.
FIGURE 2: Separation of ibuprofen, valerophenone (internal standard), and an ibuprofen-related compound C using a Lucidity miniLC. Per published monograph (10), concentrations were 12 mg/mL, 0.35 mg/mL, and 0.012 mg/mL, respectively. A 4.6 x 100 mm C18 column was operated at 1.5 mL/min for the isocratic separation, using 40:60 water:acetonitrile (+0.4% chloroacetic acid) and a 5 μL injection volume.
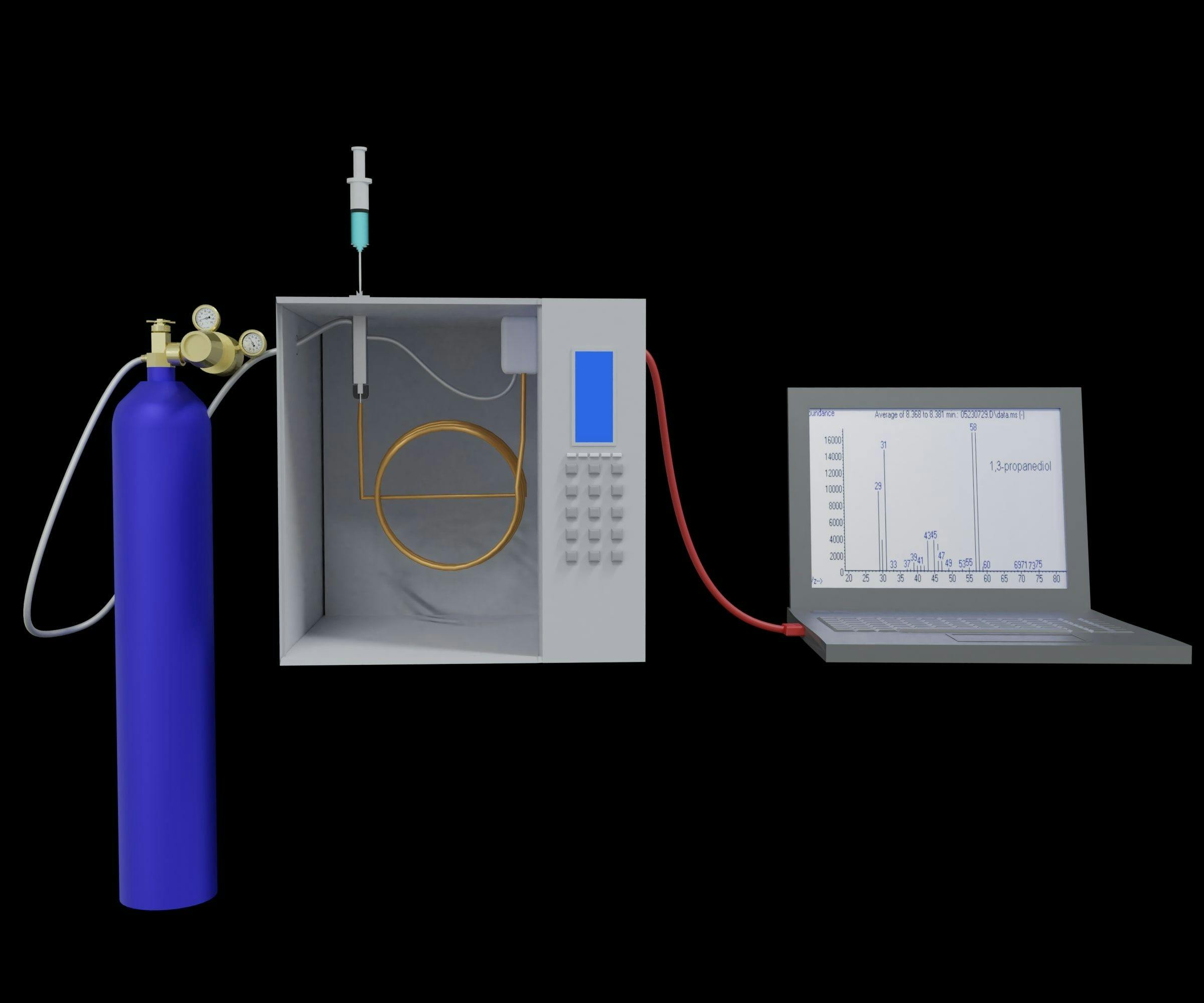
A similar miniGC system has also been released, which we are working toward implementing in our ongoing collaboration with the Department of Health & Exercise Science at Rowan University. Various research projects in this school focus on tracking plasma free fatty acid (FFA) levels in patients over time, as specific compounds can often be used as a marker of nutritional intake of specific lipids (11). Typically, samples are collected and sent to off-site testing facilities for FFA profiling by GC–MS following fatty acid methyl ester (FAME) derivatization of the extracted samples.
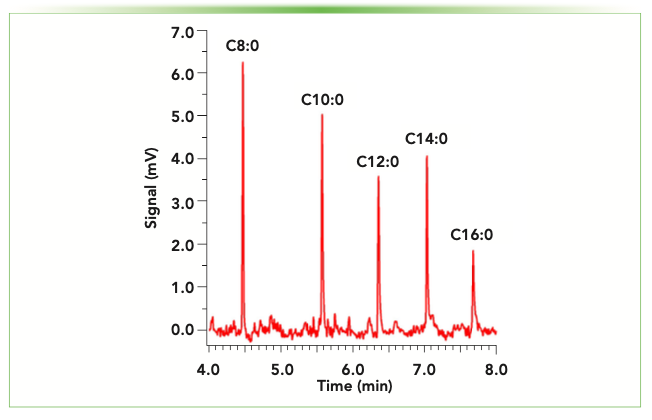
To save time and money for these measurements, which can both be limited resources in academic research settings, we are developing a GC-flame ionization detector (FID) method with the miniGC system. Preliminary results on a set of five FAME standards are shown in Figure 3. Although MS has advantages over FID in terms of sensitivity and mass identification, the added detector size can make it difficult to implement directly in clinical research spaces, similar to the aforementioned POC environments. To achieve the benefits of both approaches, collected samples can be tested twice: once with the miniGC system directly after the sample is obtained for rapid preliminary screening and later with more routine GC–MS methods in a testing laboratory for confirmation of results. As with the miniLC system, this is one of several areas in which we anticipate using the compact GC instrument, and it is likely that both will also be integrated into our undergraduate curriculum as part of our analytical laboratory activities.
FIGURE 3: Separation of five fatty acid methyl esters (labeled with free fatty acid designation) using a Lucidity miniGC. Compounds were each 1 ppm, with 4 μL injection and 5:1 split ratio. A 30 m Restek MXT-5 column (0.250 mm i.d., 0.25 μm film thickness) was used with a 120–280 oC temperature ramp at 35 oC/min (helium carrier gas, 2 mL/min, 2 min hold at initial and final temperature).
These projects utilizing various compact chromatographic instruments provide just a few examples of ways that such systems can be implemented. There are many applications related to environmental analysis, manufacturing process monitoring, health screening, food and beverage analysis, and more that are being developed on both the systems described here and other small LC, GC, and capillary electrophoresis (CE) instruments that are also in development or already commercially available. Portable and compact spectroscopic devices have advanced tremendously over the past few decades (12), and the potential for the separation science community to pursue a similar path in coming years is becoming increasingly more viable as miniaturized technology continues to advance.
Acknowledgments
We are grateful to Prof. Dylan Klein (Rowan University) and the teams at Axcend and Lucidity for ongoing collaborations related to the various projects described here.
References
(1) J.P. Grinias, LCGC North Am. 6S(38), 15–24 (2020).
(2) M.L. Lee et al., LCGC North Am. 38(7s), 12–14 (2020).
(3) J. Edelman, LCGC Column 16(10), 2–4 (2020).
(4) N.H. Snow, LCGC North Am. 39(3), 128–131 (2021).
(5) Substance Abuse and Mental Health Services Administration, Clinical Drug Testing in Primary Care. Technical Assistance Publication (TAP) 32. HHS Publication No. (SMA) 12-4668. (2012).
(6) National Institute on Drug Abuse, Overdose Death Rates (2021). <https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates>
(7) S.W. Foster et al., J. Sep. Sci. 43(9–10), 1623–1627 (2020).
(8) G.A. Kresge, J.-M.T. Wong, M. De Pra, F. Steiner, and J.P. Grinias, Chromatographia 82(1), 465–475 (2019).
(9) G.A. Kresge et al., J. Sep. Sci. 43(15), 2964–2970 (2020).
(10) United States Pharmacopeial Convention, United States Pharmacop. Natl. Formul. (USP 40-NF 35), 4555–4556 (2017).
(11) J. Ren et al., Curr. Pharm. Anal. 9(4), 331–339 (2013).
(12) R. Crocombe, P. Leary, and B. Kammrath, Portable Spectroscopy and Spectrometry (Wiley, Hoboken, NJ, 2021).
ABOUT THE AUTHORS
Sangeeta Kurre, Samuel Foster, Kyle Morrow, Alexis Zimmer, Mita Ray, Leah Notarfrancesco, Keyur Patel, and James P. Grinias are with the Department of Chemistry & Biochemistry at Rowan University, in Glassboro, New Jersey. Direct correspondence to: grinias@rowan.edu.

Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.