Acid-Catalyzed Isomerization of Carbonyls-2,4- dinitrophenylhydrazone in Mainstream Smoke of Heat-Not-Burn Tobacco Product for HPLC Analysis
Carbonyls (such as acrolein, acetaldehyde, and formaldehyde) are the critical type of carcinogens and toxicants contained within the heat-not-burn (HNB) tobacco products. Using HNB products can have negative effects on human health; therefore, it is important to measure carbonyl contents within the HNB mainstream smoke. Typically, the 2,4-dinitrophenylhydrazine (DNPH) approach involves forming the 2,4-dinitrophenylhydrazone derivatives, which is the most extensively adopted approach to qualitatively and quantitatively analyze carbonyl compounds. However, the approach can result in analytical error because 2,4-dinitrophenylhydrazones contains the E-stereoisomer as well as the Z-stereoisomer. Only an E-isomers exists in the purified carbonyls-2,4-dinitrophenylhydrazone, but when acid is added, the E-isomer and Z-isomer can be observed. For propionaldehyde-, acetaldehyde-, crotonaldehyde-, acrolein-, and 2-butanone-2,4-dinitrophenylhydrazones, their equilibrium Z/E isomer ratios are 0.143, 0.309, 0.093, 0.028, and 0.154. In the case of adding trace water into hydrazone derivatives dissolved within the acetonitrile solution, the derivative contents decrease, whereas the free DNPH content increases. Therefore, catalytic acid should be added in the low content. To determine carbonyls-2,4-dinitrophenylhydrazones through HPLC, the optimal approach is adding phosphoric acid into the samples and the standard reference solution to form the 0.02–1.0% acid solution.
Smoking increases the risk of humans contracting various diseases and even death. Therefore, numerous smokers have begun to search for tobacco products that have decreased health risks. According to the definition from the Family Smoking Prevention and Tobacco Control Act 2009, the “modified risk tobacco product (MRTP) is the tobacco product distributed and sold aiming to decrease the risks of harm and tobacco-associated disorders (1).” MRTPs for injury reduction is specifically targeted at smokers who are unwilling or unable to quit smoking. As a MRTP candidate, the Tobacco Heat- ing System (THS) 2.2 (marketed as IQOS) is one of the heat-not-burn (HNB) tobacco products that are distinguished from traditional “burn” tobacco by their additives and processing differences. THS 2.2 is an electrically heated cigarette. When smoking a THS 2.2, the cigarette is kept unburned, and the temperature is lower than the ignited end. As a result, such products heated by electricity can decrease pyrolysis, leading to the decreased contents of harmful and potentially harmful constituents (HPHCs) within the produced smoke (2–7).
Cigarette smoke is a complicated mixture constituted by over 5000 identified chemicals, which can be classified as particulate matter (PM) and gas phase matter; typically, over 50 of these chemicals are carcinogenic (8–10). In the monographs by the International Agency for Research on Cancer (IARC) (11), the harmful substance carbonyl compounds, including ketones and aldehydes contained within the cigarette smoke, aroused wide attention in the biochemistry and environmental chemistry fields. Chronic exposure to the formaldehyde content poses an increased risk to human beings (12–15). In 2004, formaldehyde was reclassified by the IARC as a class 1 human carcinogen that may cause nasopharyngeal carcinoma, and it was stated that occupational formaldehyde exposure is evidently related to leukemia (16). Additionally, acetaldehyde can lead to higher risks of esophageal, head, and neck cancer (17–19). Acrolein is not suspected as a human carcinogen because no existing study has reported that it can induce carcinogenicity of human cells. Nonetheless, rat studies discovered that ingestion, not inhalation, will lead to an increased risk of cancerous tumors. According to research in recent years, acrolein in cigarette smoke is associated with an increased risk of lung cancer (20).Therefore, it is significant to measure carbonyl concentration within HNB smoke and to assess how HNB tobacco products affect human health. Carbonyl compounds are among those major toxicants within the HNB smoke. According to the Food and Drug Administration (FDA) in the United States, eight carbonyl compounds, including acetaldehyde, formaldehyde, acetone, acrolein, crotonaldehyde, propionaldehyde, 2-butanone, and butyraldehyde, are listed among the 93 harmful and potentially harmful components (HPHCs). Currently, the most widely adopted approach to determine ketones and aldehydes is to form the stable Schiff bases (C=N) according to the reaction between the amino (NH2) fraction and the carbonyl (C=O) counterpart. As far as high performance liquid chromatography (HPLC) analysis is concerned, derivatization plays an important role. For over 70 years, ketones and aldehydes have been known to react with 2,4-dinitrophenylhydrazine (DNPH) for the formation of relevant hydrazone derivatives. Once formed, the derivatives are separated by HPLC, and an UV detection wavelength is selected for the maximum absorption of hydrazone (usually 360 nm), as first reported by Allen (21) and Brady (22). For the DNPH approach, its major merit lies in its capacity to simultaneously analyze different ketones and aldehydes within the complicated mixture. The DNPH acid solution in the impinging sampler can be used for sampling (23), or the acid DNPH-coated Cambridge filter can be used for sampling. Because of the importance of this method, some national standardization protocols have introduced it as a standard procedure, even though isomer analysis is difficult (24,25). Although the formation of the isomer 2,4-dinitrophenylhydrazone is well-known, hardly anyone has noticed that there may be some analytical issues in determining ketones and aldehydes within the HNB smoke. In particular, when smoke samples are prepared, the occurrence or extent of isomerization is unclear.
Uchiyama and others (26–28) elucidated the isomerization mechanism of 2,4-dinitrophenylhydrazone. E-isomer alone was contained in aldehyde-2,4-dinitrophenylhydrazone after purification. However, both E- and Z-isomers appeared under UV irradiation and acid addition. As suggested by such findings, the addition of phosphoric acid to the standard solution and sample for the formation of a 0.003–0.15 mol/L acid solution is the optimal approach to determine aldehyde-2,4-dinitrophenylhydrazine using HPLC.
Therefore, the reaction between aldehydes and DNPH has been studied, but almost nothing is known about the simultaneous detection of acetaldehyde, formaldehyde, acetone, acrolein, crotonaldehyde, propionaldehyde, 2-butanone, and butyraldehyde within the smoke. The present work aimed to examine the impacts of adding phosphoric acid and water on isomerization and decomposition, respectively, of the above-mentioned eight carbonyl derivatives. The HPLC approach was put forward to determine carbonyl-2,4-dinitrophenylhydrazone within the HNB mainstream smoke.
Materials and Methods
Instruments
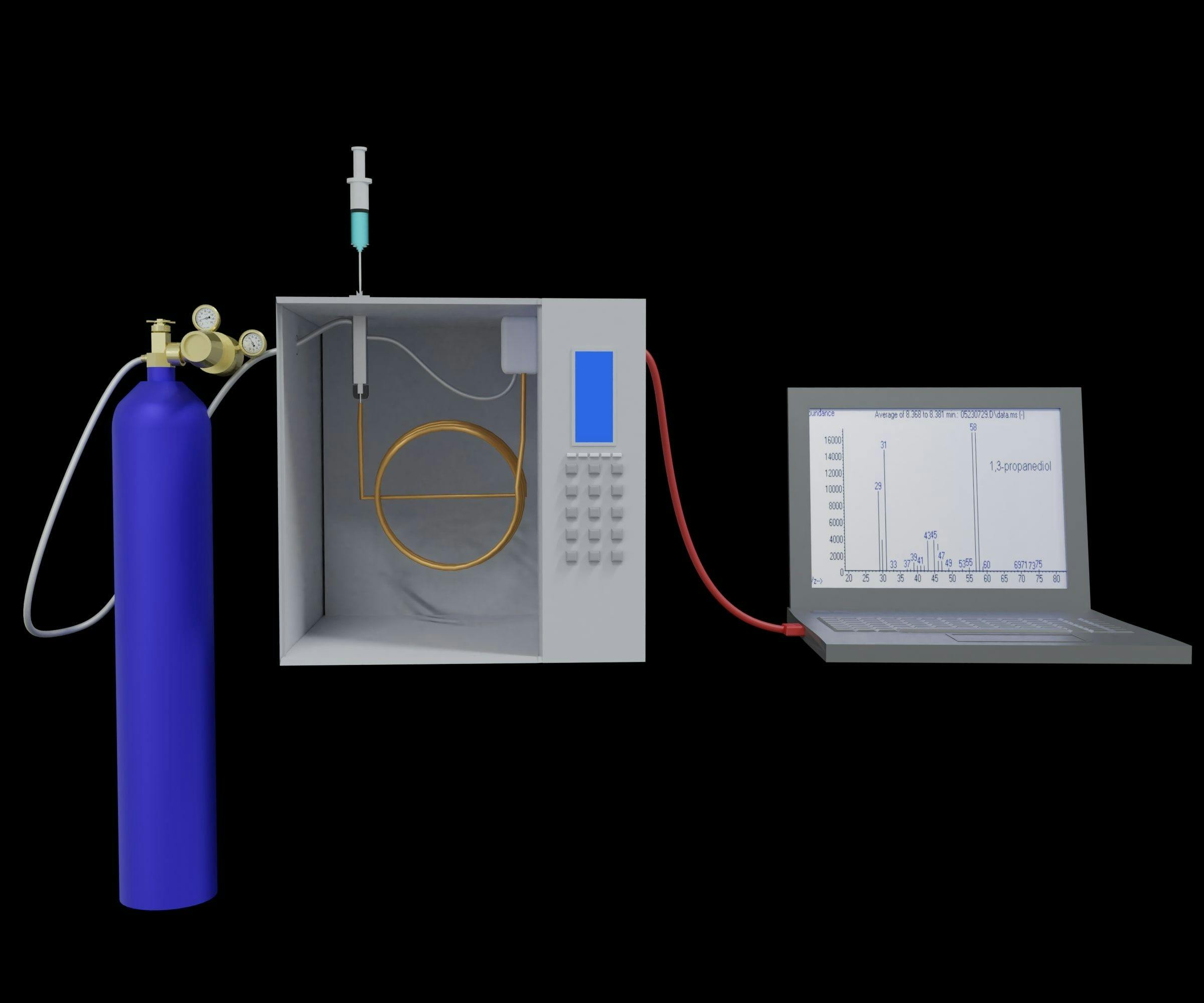
The HPLC system consisted of a 1290 Infinity LC system (Agilent Technologies) with a diode array detector. A 25 × 0.46 cm (i.d.) Acclaim Explosives E2 column was provided by Thermo Fisher Scientific Inc. An SM450 linear smoking machine from Cerulean was used for collecting carbonyls in the HNB mainstream smoke.
Reference Substances and Reagents
Deionized water was further purified by the Milli-Q water system (Millipore) and was utilized in HPLC and in preparing samples. Phosphoric acid and acetonitrile (85%, w/v, dissolved within water) were provided by Chemicell Scientific. The 2,4-dinitrophenylhydrazine was obtained from Chemicell Scientific, which was subjected to recrystallization within acetonitrile prior to use in order to remove water. The standard 2,4-dinitrophenylhydrazone derivatives of the above-mentioned eight carbonyl substances (including acetaldehyde, formaldehyde, acetone, acrolein, crotonaldehyde, propionaldehyde, 2-butanone, and butyraldehyde) were synthesized by Sigma–Aldrich. The Cambridge glass fiber filter (44 mm diameter) used was from Cerulean. The Tobacco sheet and THS 2.2 systems were obtained from Philip Morris International.
Measurement of E- to Z-Isomerization with Phosphoric Acid
For this study, 1 mL of the 1 mmol/L carbonyls-2,4-dinitrophenylhydrazone was made within the 10 mL volumetric flask. For preparing the 10−7−10−1 mol/L acetonitrile/phosphoric acid solutions; we added aliquots of the 0.001, 0.01, 0.1, and 1 mol/L acetonitrile–phosphoric acid solutions, followed by immediate dilution with acetonitrile to 10 mL volume. At 0.16, 22, 40, 52, and 100 h later, the HPLC was adopted to analyze the solutions using the autosampler at 25 °C. Thereafter, the isomer ratio was determined based on the peak area at the maximum spectrum.
Decomposition of Carbonyls-2,4-Dinitrophenylhydrazones
For this study, 1 mL carbonyl-2,4-dinitrophenylhydrazine (1 mmol/L) flask was prepared in a 10 mL capacity. To be specific, the 1 mol/L acetonitrile/phosphoric acid solutions were mixed with 0, 100, 200, 500, or 1000 μL water, followed by immediate dilution with acetonitrile to 10 mL, yielding the water content of 0.056, 6.1, 12, 28, or 56 mmol/L, respectively. Later, HPLC was conducted to analyze the solutions under 25 °C.
HNB Mainstream Smoke Carbonyls Isomers Analysis
Before smoking, the consumable cigarette stick is adjusted to 22 °C and 60% RH for at least 48 h before smoking. The SM450 linear smoking machine was employed for smoking. The automatic machine was utilized in the THS 2.2 system for heating the expendable cigarette stick for approximately 6 min by pressing the start button after ignition. In the working state, the cigarette is controlled in the non combustion state under the heating state, and the length of consumables remains unchanged after smoking, which makes the wind speed control inaccurate.
The smoke was produced in accordance with the International Organization For Standardization (ISO; 35 mL blow, 2 s duration, 60 s interval) and Canadian Department of Health intensive (HCI; 55 mL blow, 2 s duration, 30 s interval, ventilation blockage) methods (29,30).
Using a Cambridge filter coated with DNPH solution, the carbonyl compounds in the mainstream smoke of five consumable cigarette sticks were determined by HPLC. Approximately 1.5 g of recrystallized DNPH was dissolved in 100 mL acetonitrile and 70 μL phosphoric acid to prepare the DNPH solution. The two Cambridge filters were put together, and 2 mL of the DNPH solution was added and then left for 30 min before smoking. After smoking, the Cambridge filter was transferred to a scintillation vial, and 50 mL acetonitrile containing 2% pyridine was added to extract carbonyl compounds, and stored at 4 °C for HPLC diode array detection (DAD) analysis.
A 25 × 0.46 cm (i.d.) Acclaim Explosives E2 column was used. The eluent contained water and acetonitrile. For the mobile phase, the injection volume and flow rate were 10 μL and 1.5 mL/min, respectively. The diode array detector was set at 365 nm. An optimized gradient for isomer separation of acetonitrile–water was employed as follows: (water [A] and acetonitrile [B]): Start with 50% v/v A, then go up to 20 min 50% v/v A, up to 25 min to 40% v/v A, up to 30 min to 40% v/v A, up to 35 min to 20% v/v A, up to 40 min 10% v/v A, up to 41 min to 50% v/v A, and finally, up to 45 min 50% v/v A.
Results and Discussion
Optimization of the Isomer Separation Condition
The analytical conditions of E- and Z-isomers of carbonyl-2,4-dinitro- phenylhydrazone were investigated by three different HPLC columns. In HPLC, it is difficult to separate the E-isomer from the Z-isomer in the carbonyl-2,4-dinitrophenylhydrazone. Figures 1a and 1b show that the two isomers were successfully separated using an Explosive E2 column. It is based on high-purity silica and an alkyl amide reversed-phase column. Compared to the simple hydrophobic affinity of the C18 alkyl, the presence of alkyl amide group makes the column have a different selectivity. Two retention mechanisms may be responsible for the presence of alkyl along with alkyl amide groups in the E2 column. The first one is related to the affinity for hydrophobic alkyl, whereas the second one is related to the interaction of amide groups with the polar groups in 2,4-dinitrophenylhydrazine derivatives. Therefore, the E2 column has unique selectivity and applicability, which allows the E- and Z-isomers of 2,4-dinitrophenylhydrazine derivatives to have enough separation. The isomers of carbonyl-2,4-dinitrophenylhydrazone were completely separated in the chromatogram generated by the E2 column.
FIGURE 1a: The chromatograms of the standard solution.
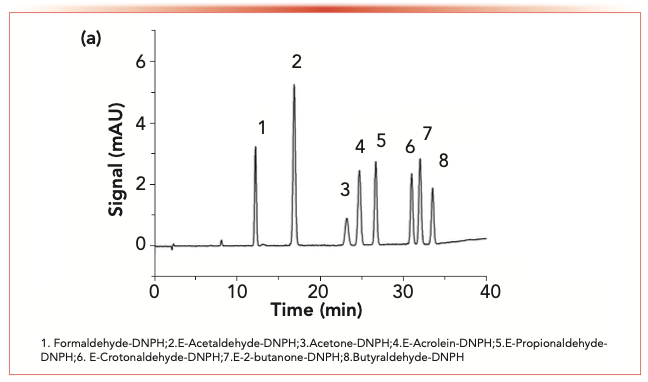
FIGURE 1b: The chromatograms of the representative HNB mainstream smoke of carbonyls-2,4-dinitrophenylhydrazone.
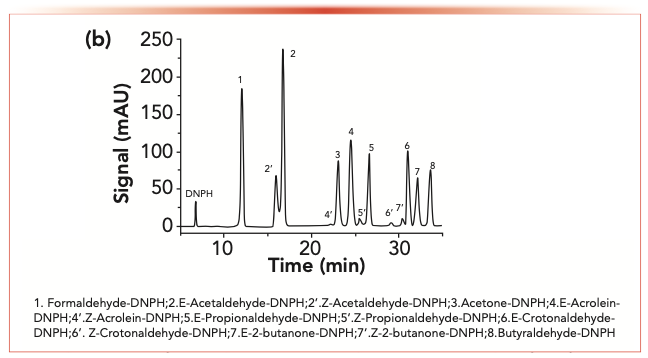
No Z-isomer was detected in the purified carbonyl-2,4-dinitrophenylhydrazone. Nonetheless, when little phosphoric acid was added, isomerization occurred. The chromatograms for the phosphoric acid sample and acid-free standard solutions of eight carbonyl-2,4-dinitrophenylhydrazine derivatives are shown in Figure 1. The Z- and E-isomer peaks were observed in the acid solution, whereas only E-isomer peaks were seen in the acid-free solution.
UV–Vis Spectra of Isomers
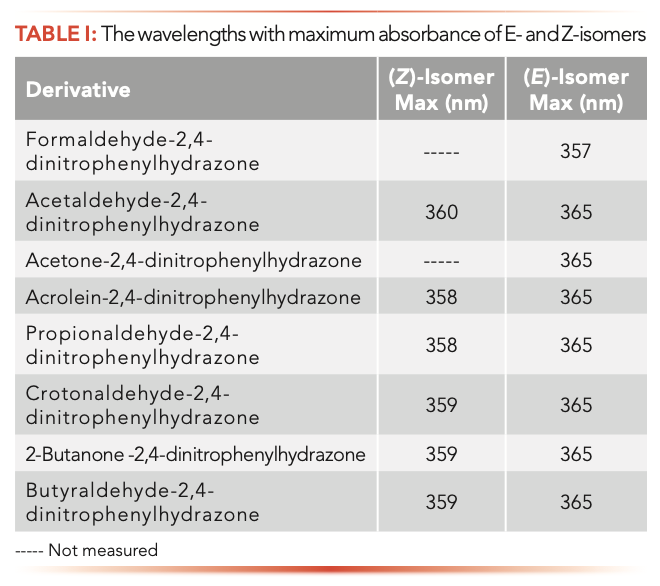
Figure 2 shows the UV-vis spectra of the E- and Z-isomers of acetaldehyde-2,4-dinitrophenylhydrazine. The E-isomer showed distinct spectral profiles and maximal absorption wavelength from those of the Z-isomer (at 365 and 360 nm, separately). For the remaining carbonyl-2,4-dinitrophenylhydrazone isomers, they showed similar spectral distribution to the acetaldehyde-2,4-dinitrophenylhydrazone isomer. Compared with the E-isomer, the Z-isomer had the maximal absorption wavelength shifted to a shorter wavelength by 5–8 nm (Table I) (26,27).
FIGURE 2: UV spectrum of acetaldehyde-2,4-dinitrophenylhydrazone E- and Z-isomers.
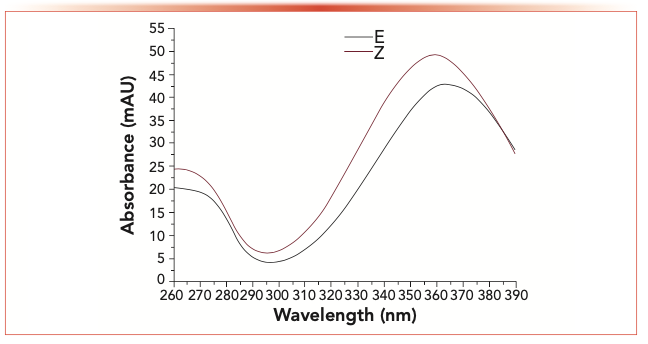
The E-isomer and Z-isomer contents should be used to obtain the precise isomer ratio. Nonetheless, the concentration of Z-isomer cannot be determined due to the absence of standard reagents. As a result, we defined isomer ratio as the ratio of peak area, which was determined based on the maximal wavelength of E-isomer (365 nm).
E to Z Isomerization with Phosphoric Acid
It was stated in a previous section that the Z-isomer was not observed in the purified carbonyl-2,4-dinitrophenylhydrazone; by contrast, isomerization may be detected when trace phosphoric acid was added or under UV radiation conditions. The alterations in isomer ratios for the carbonyl-2,4-dinitrophenylhydrazone derivatives under varied contents of phosphoric acid are presented in Figure 3. In the presence of 0.0001% phosphoric acid, a small amount of isomerization was observed, whereas 0.004% phosphoric acid significantly increased the isomer ratio. The equilibrium isomer ratio of acetaldehyde-2,4-dinitrophenylhydrazine was about 0.31. At lower acid concentration, the equilibrium isomer ratio reached 0.31 with the extension of reaction time. After 22 h of reaction, the identical curve associated with the equilibrium isomer ratio was seen within the phosphoric acid concentration range of 0.001–0.005%.
FIGURE 3a: The changes of the isomer ratios with phosphoric acid.
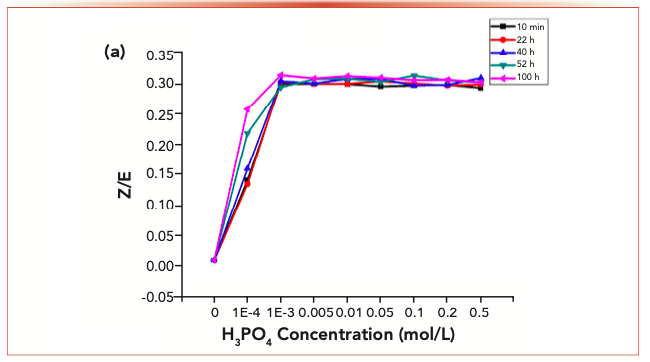
FIGURE 3b: The changes of the isomer ratios with phosphoric acid.
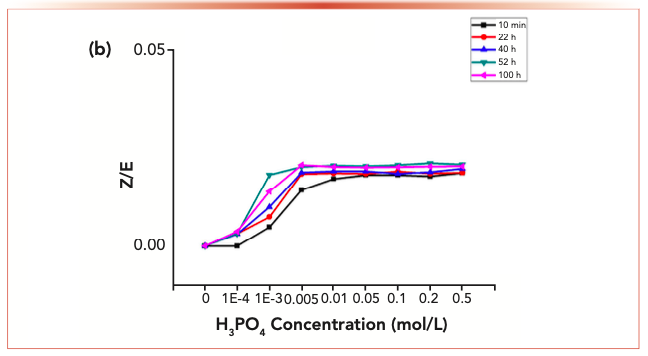
FIGURE 3c: The changes of the isomer ratios with phosphoric acid.
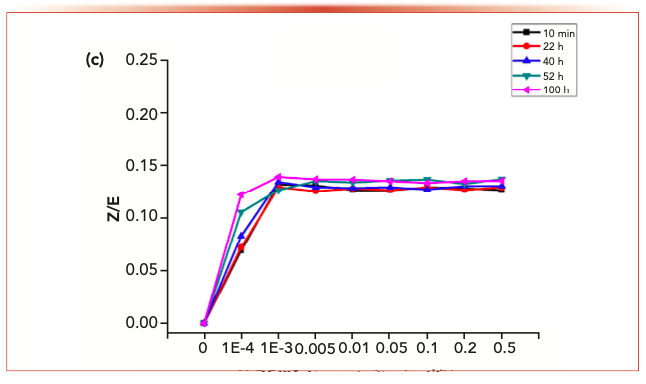
FIGURE 3d: The changes of the isomer ratios with phosphoric acid.
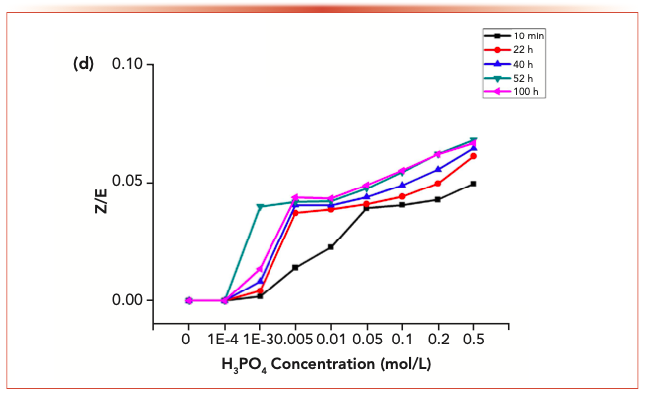
FIGURE 3e: The changes of the isomer ratios with phosphoric acid.
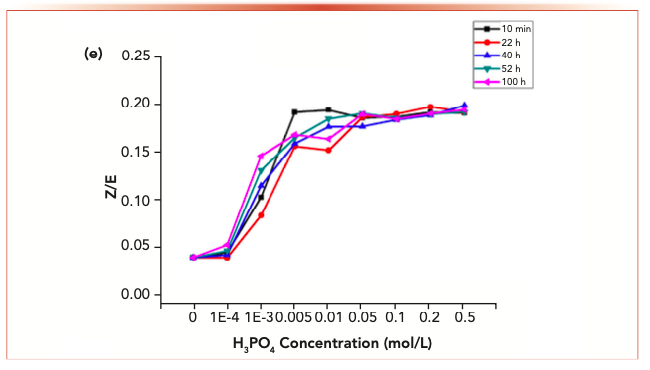
Acrolein-2,4-dinitrophenyl hydrazone, propionaldehyde-2,4-dinitrophenyl hydrazone, crotonaldehyde-2,4-dinitrophenylhydrazone and 2-butanone-2,4-dinitrophenylhydrazone derivatives displayed similar behaviors. For each reaction system with the addition of phosphoric acid, no reduced content because of degradation was detected.
The isomerization of protonated carbonyl-2,4-dinitrophenylhydrazones within the acidic solution probably took place by the reaction shown in Visual A.
VISUAL A: Proposed isomerization of protonated carbonyl-2,4- dinitrophenylhydrazones within the acidic solution.
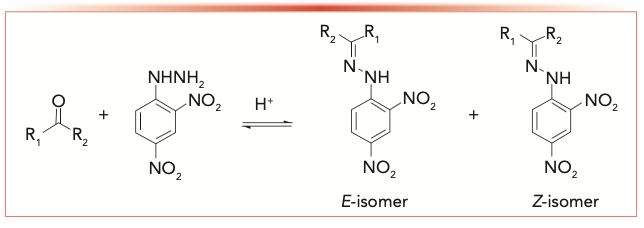
The phosphate ion served as the catalyst to isomerize carbonyl-2,4-dinitrophenylhydrazone, which was not involved in the equilibrium isomer ratio constant. The effect of alkyl substituents on the isomerization rate is very small. Other 2,4-dinitrophenylhydrazine derivatives exhibit similar behavior to acetaldehyde-2,4-dinitrophenylhydrazine and have similar equilibrium isomer ratios (Table II). The isomer ratio of butyraldehyde-2,4-dinitrophenylhydrazine cannot be determined by HPLC.
Decomposition of Carbonyl S-2,4-Dinitrophenylhydrazones with Phosphoric Acid and Water
Carbonyl substances, such as ketones, can form hydrazones through the reaction with DNPH (31). In this reaction, acid at the catalytic dose is needed. Besides, the protonated hydrazone can be formed from oxygen protonated intermediate by losing a proton after removing water. The hydrazone formation process can be reversible. Hydrazone derivatives within acidic water solution can be hydrolyzed back to produced DNPH and carbonyl substances; later, the reaction equilibrium can be reached. Phosphoric acid plays a catalytic role in increasing the reaction rate, but does not affect the equilibrium constant.
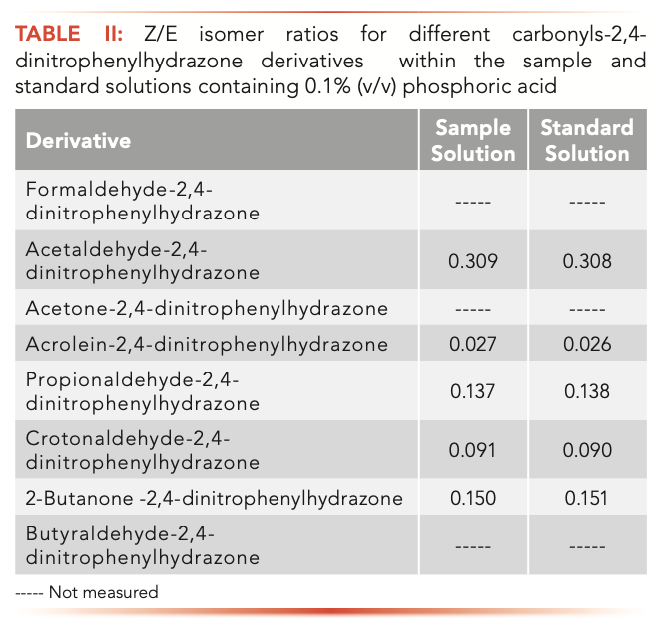
In the isomerization reaction, the concentration of the derivatives decreased because of the degradation and formation of DNPH. Figure 4 shows the decomposition process of eight carbonyl 2,4-dinitrophenylhydrazone in phosphoric acid. The results show that with the increase of phosphoric acid concentration, only acetone-2,4-dinitrophenylhydrazone and 2-butanone-2,4-dinitrophenylhydrazone continuously degrade; acetaldehyde-2,4-dinitrophenylhydrazone only degrades at very low concentration of phosphoric acid, and then remains stable with the increase of phosphoric acid concentration.
FIGURE 4: Decomposition of 8 carbonyls 2,4-dinitrophenylhydrazones with phosphoric acid.
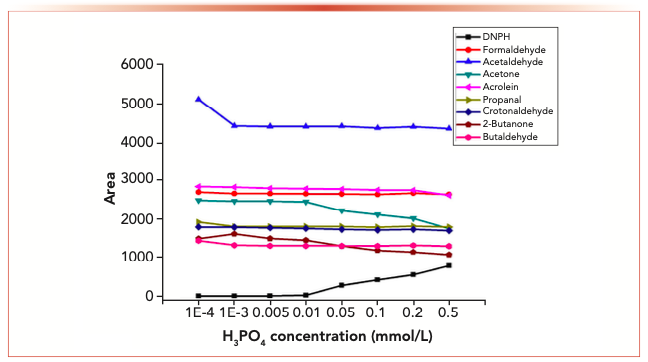
Figure 5 shows the decomposition reaction of eight carbonyl 2,4-dinitrophenylhydrazones with water. As suggested by these findings, continuous degradation was only observed in acetone and 2-butanone 2,4-dinitrophenylhydrazones as the water content increased; other aldehydes-2,4-dinitrophenylhydrazone remained stable with the increase of water concentration; DNPH concentration increased significantly with the increase of water concentration. The results show that the decrease of derivative concentration is because of the presence of H2O in phosphoric acid. For hydrazone derivatives of aldehydes, at all experimental conditions, aldehyde-2,4-dinitrophenylhydrazone remained undegraded.
FIGURE 5: Decomposition of 8 carbonyls 2,4-dinitrophenylhydrazones with water.
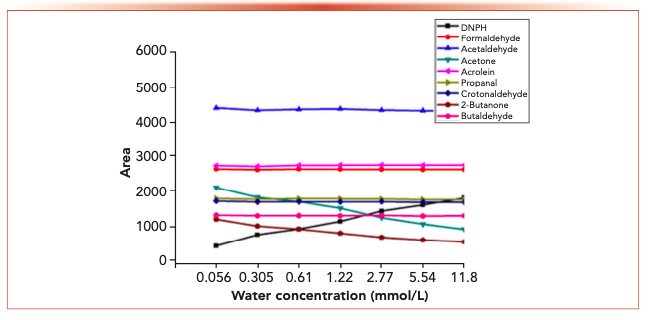
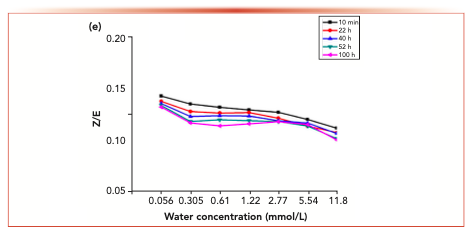
Figure 6 shows the isomer changes of acetaldehyde-2,4-dinitrophenyl hydrazone, acrolein-2,4-dinitrobenzene hydrazone, propionaldehyde-2,4-dinitrophenyl hydrazone, crotonaldehyde-2,4-dinitrobenzone hydrazone and 2-butanone-2,4-dinitrophenylhydrazone derivatives in different aqueous solutions. The results showed that the Z/E ratio of 2-butanone-2,4-dinitrophenylhydrazone decreased with the increase of water concentration, whereas the Z/E ratio of aldehyde-2,4-dinitrophenylhydrazone remained stable with the increase of water concentration.
FIGURE 6: The changes of the isomer ratios with water concentration.
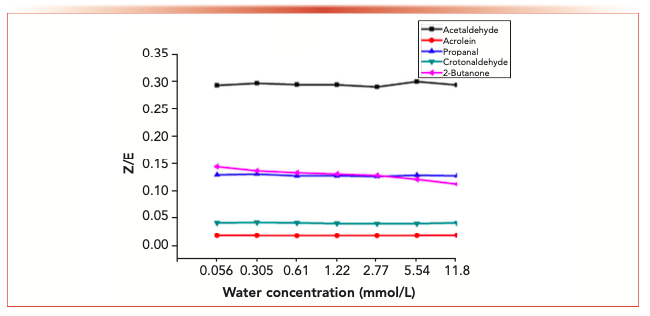
Figure 7 shows the variation of isomer ratios of acetaldehyde-2,4-dinitrophenylhydrazone, acrolein-2,4-dinitrophenylhydrazone, propionaldehyde-2,4-dinitrophenylhydrazone, butyraldehyde-2,4-dinitrophenylhydrazone derivatives and 2-butanone-2,4-dinitrophenylhydrazone derivatives over time. The results showed that the Z/E ratio of 2-butanone-2,4-dinitrophenylhydrazone decreased with time, whereas aldehyde-2,4-dinitrophenylhydrazone remained stable.
FIGURE 7a: The changes in the isomer ratios with time at different water concentration.
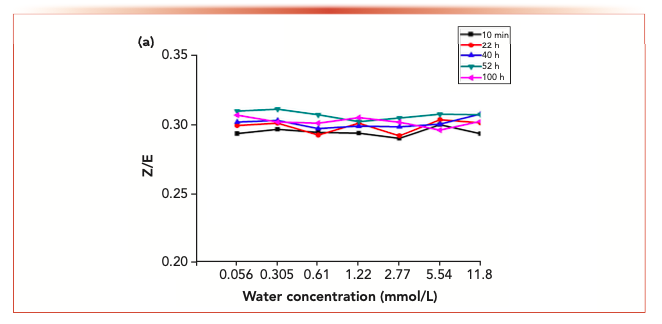
FIGURE 7b: The changes in the isomer ratios with time at different water concentration.
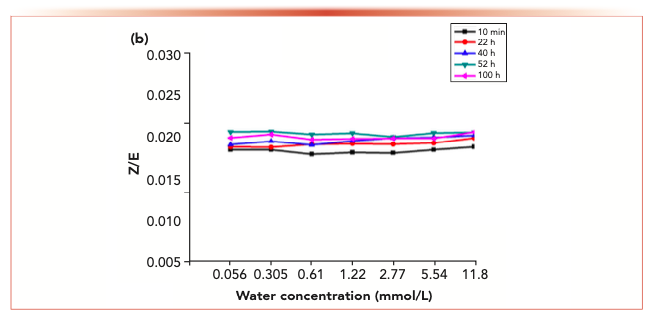
FIGURE 7c: The changes in the isomer ratios with time at different water concentration.
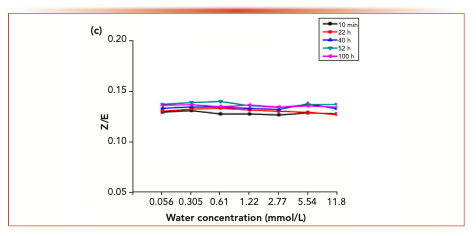
FIGURE 7d: The changes in the isomer ratios with time at different water concentrations.
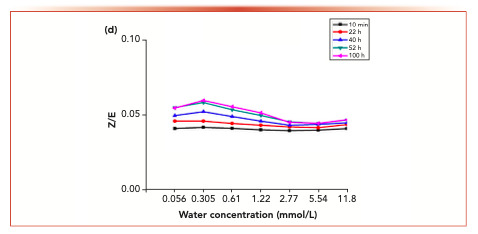
FIGURE 7e: The changes in the isomer ratios with time at different water concentrations.
Results of Carbonyls Isomers in HNB Mainstream Smoke
When the mainstream smoke sample is extracted through a DNPH coated filter, carbonyl groups are derived. The filter-coated DNPH contained phosphoric acid, which was the essential catalyst used to catalyze the reaction between the DNPH and carbonyl substances. The sample derivation process is an acidic solution. Therefore, E- and Z-isomers were observed simultaneously when the extract was analyzed by HPLC as shown in Figure 1.
According to reference (32), the equilibrium of Z/E-isomerization may be affected by solvents, especially trace acids. Therefore, the single isomer peak area can only be used to quantify the results in the case of identical conditions for sample preparation, smoke sampling, along with calibration method. We concluded the peak areas for Z- and E-isomers briefly. The correct results can be obtained only when the molar extinction coefficients of the two isomers are equal at the selected wavelength. According to the peak area of the compounds studied, the calibration curve shows linearity and reproducibility, which was not affected by the isomer ratio and sampling conditions. It was dis- covered that each compound had similar or equal extinction coefficients of both isomers. As a result, for the eight carbonyl 2,4-dinitrophenylhydrazine substances, their total peak areas might be used to calibrate and quantify the results, which was not affected by the diverse sampling conditions.
Table III shows the transfer of carbonyl isomers in HNB mainstream smoke under ISO and HCI smoking protocols. Our results show that the release ratio of carbonyl isomers of HNB is similar under two different machine smoking methods (ISO and HCI). We found that the release of acetaldehyde from HNB was the highest by ISO and HCI, followed by acetone and formaldehyde. Because acetaldehyde is the most abundant carcinogen in cigarette smoke, and the Z/E isomer ratio of acetaldehyde under ISO method is the largest (0.309), this discovery is of great significance. Similar to acetaldehyde, the Z/E isomer ratios of propionaldehyde and 2-butanone are higher, which are 0.143 and 0.154 respectively under the ISO method. Acrolein is a serious respiratory and cardiovascular toxicant. The Z/E isomer ratios of acrolein were 0.028 and 0.027, respectively. Similar to acrolein, the Z/E isomer ratio of crotonaldehyde is low, which is 0.093 and 0.090 respectively by the ISO method and HCI method.
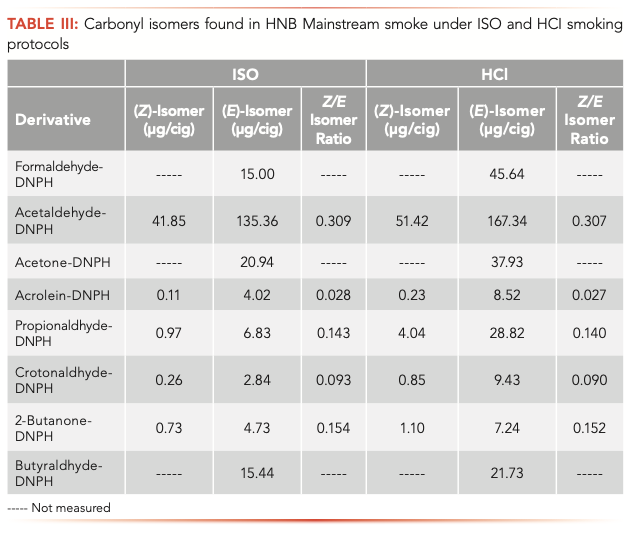
These findings suggest that both Z- and E-isomers should be regulated if FDA regulates the carbonyl transfer of HNB. This information helps to guide FDA regulation of HNB, in particular for formulating suitable product standards and warning the possible toxic effect on exposing to such substances to the public.
Conclusion
The formation of Z and E isomers of 2,4-dinitrophenylhydrazone may cause analysis problems when a 2,4-dinitrophenylhydrazine coated filter is used to determine carbonyls in HNB mainstream smoke. The purified 2,4-dinitrophenylhydrazine derivatives contained only E-isomer. Nonetheless, part of E-isomer is transformed to Z-isomer following the addition of a catalytic acid. As a result, adding phosphoric acid at an identical dose into the standard solution is necessary for the formation of identical isomer ratio to that of sample solution. The ketone hydrazone derivatives will be hydrolyzed back into DNPH and carbonyl substances in the acidic water solution. In this regard, catalytic acid at the lowest dose should be adopted.
Only when the proportion of isomers is constant, it is possible to calculate the peak areas of the two isomers, such as the maximum absorption of E- and Z-isomers at different wavelengths. When the total peak area is not dependent on isomer ratio, it is regarded as a proof. To conclude, the optimal approach to determine carbonyl-2,4-dinitrophenylhydrazone through HPLC is adding the phosphoric acid to standard and sample solutions for the formation of acid solutions at a dose of 0.02–1.0%.
References
(1) Family Smoking Prevention and Tobacco Control Act, Pub. L. No. 111–131 (2009).
(2) X.Y. Li, Y.B. Luo, X.Y. Jiang, H. Zhang, F. Zhu, S. Hu, et al., Nicotine Tob. Res. 21(1),111–118 (2019).
(3) J.P. Schaller, J.P.M. Pijnenburg, and A. Ajithkumar, Regul. Toxicol. Pharm. 81, 48–58 (2016).
(4) J.P. Schaller and D. Keller, Regul. Toxicol. Pharm. 81, 27–47 (2016).
(5) G. Patskan, W. Reininghaus, J. Appl. Toxicol. 23, 323–328 (2003).
(6) M.W. Ogden, K.M. Marano, B.A. Jones, W.T. Morgan, and M.F. Stiles, Biomarkers 20, 391–403 (2015).
(7) E. Roemer, H. Schramke, and H. Weiler, Beiträge Zur Tabakforschung 25(1), 316–335 (2014).
(8) S. Uchiyama, T. Tomizawa, Y. Inaba, and N. Kunugita, J. Chromatogr. A. 1314, 31–37 (2013).
(9) A. Thielen, H. Klus, and L. Muller, Exp. Toxicol. Pathol. 60, 141–156 (2008).
(10) M. Borgerding and H. Klus, Exp. Toxicol. Pathol. 57(Suppl1), 43−73(2005).
(11) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 340(Suppl. 7), 120–122 (1987).
(12) R. Golden, D. Pyatt, and P.G. Shields, Crit. Rev. Toxicol. 36, 135–153 (2006).
(13) T. Salthammer, S. Mentese, and R. Marutzky, Chem. Rev. 110, 2536–2572 (2010).
(14) R. McConnell, T. Islam, K. Shankardass, M. Jerrett, F. Lurmann, and F. Gilliland, Environ. Health Perspect. 118(7), 1021–1026 (2010).
(15) National Toxicology Program, Final Report on Carcinogens Background Document For Formaldehyde, 10–5981, i–512 (2010).
(16) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 88, 39–325 (2006).
(17) S. Boccia, M. Hashibe, and P. Gallì, Biomarkers Prev. 18, 248(2009).
(18) K. Kida, H. Oda, Y. Yamano, and J. Kagawa, Eur. Respir. J. 10, 2124–2126 (1997).
(19) H. Kenche, Z.W. Ye, and K. Vedagiri, J. Biol. Chem. 291, 4763–4778 (2016).
(20) M. Hristova, P.C. Spiess, D.I. Kasahara, Am. J. Respir. Cell. Mol. Biol. 46, 23–33 (2012).
(21) C.F.H. Allen, J. Am. Chem. Soc. 52, 2955−2959 (1930).
(22) R.J. Kieber and K. Mopper, Environ. Sci. Technol. 24, 1477−1481 (1990).
(23) D. Grosjean, Environ. Sci. Technol. 16, 254–262(1982).
(24) M. Pawlowska and D.W. Armstrong, Chirality 6, 270–276(1994).
(25) D.W. Armstrong, M. Gasper, S.H. Lee, J. Zukowski, and N. Ercal, Chirality 5, 375–378 (1993).
(26) S. Uchiyama, M. Ando, and S. Aoyagi, J. Chromatogr. A. 996, 95–102 (2003).
(27) S. Uchiyama, T. Kaneko, H. Tokunaga, M. Ando, and Y. Otsubo, Anal. Chim. Acta. 605, 198–204 (2007).
(28) N. Binding, W. Müller, and U. Wittin, Fresenius J. Anal. Chem. 356, 315– 319 (1996).
(29) ISO 3308, Routine Analytical Cigarette - Smoking Machine - Definitions and Standard Conditions (International Organization for Standardization, Geneva, Switzerland, 2000).
(30) Health Canada Official method T-115, Determination of “Tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke, Ottawa, Canada (1999).
(31) P.Y. Bruice, Organic Chemistry (Prentice Hall, Englewood Cliffs, NJ, 2003).
(32) J.O. Levin, K. Andersson, C.A. Lindahl Rand Nilsson, Anal. Chem. 57, 1032–1035 (1985).
Hongfei Zhang, Fengpeng Zhu, Xiangyu Li, Yanbo Luo, Xingyi Jiang, Yongqiang Pang, Hongwei Hou, and Qingyuan Hu are with the Key Laboratory of Tobacco Biological Effects, and the Joint Laboratory of Heated Tobacco Product Safety Evaluation, at the China National Tobacco Quality Supervision and Test Center in Zhengzhou, China. Chao Chen and Wenliang Zhang are with the Shanghai New Tobacco Product Research Institute in Shanghai, China. Direct correspondence to Hongwei Hou at qsfctc@163.com, and Qingyuan Hu at huqy1965@163.com.

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)