The Evolution of Chiral Stationary Phases from HPLC to UHPLC
LCGC Europe
This article focuses on the progression that chiral stationary phases (CSPs), specifically developed for HPLC, are currently undergoing because of the pressing need of an easy switch to UHPLC.
There is a need for chiral stationary phases (CSPs) designed for high performance liquid chromatography (HPLC) to switch to enantioselective applications using ultrahigh-performance liquid chromatography (UHPLC). Although important goals have been achieved to rapidly separate achiral compounds, enantioselective LC remains solidly attached to 3-μm and 5-μm totally porous particles and pressure values in the HPLC domain. This article describes strategies aimed at immobilizing or coating well-established chiral selectors onto sub-2-μm silica particles, and aims to illustrate the potential of enantioselective UHPLC (eUHPLC) in terms of high speed, throughput and resolution.
Since the first commercially available ultrahigh-performance liquid chromatography (UHPLC) system was introduced in 2004, there has been a constant evolution of LC both in terms of instrumentation (ability to sustain high pressures and yield lower volume dispersion) and in terms of particle design, leading to a more universal use of UHPLC in routine and research applications. UHPLC offers new possibilities to the analytical chemist who can now choose between either ultrafast separations without (or with acceptable) efficiency loss or higher performance and resolution without speed gain in analysis time (1–3). Given the most recent advances in the field, it is now possible to reach efficiency values up to 300,000 N/m using columns packed with sub-2-μm particles, either totally porous or with a solid nucleus (4–5). The most commonly used reversed-phase and hydrophilic interaction chromatography (HILIC) chemistries are now available in the sub-2-μm format, allowing a scale-down of existing methods.

However, enantioselective LC is still performed on 3- and 5-μm totally porous particles, yielding pressure values in the high performance liquid chromatography (HPLC) domain. In fact, although HPLC and UHPLC C18 columns for reversed-phase separations have been extensively discussed and evaluated in literature in terms of kinetic and thermodynamic phenomena, little is known on the efficiency of enantioselective columns. Up to now, the main concern of both users and manufacturers has been a qualitative one: Analyses on a 45-min timescale or longer are considered typical in chiral separations. Is it really not possible to overcome this limitation?
Pirkle-Type Selectors Investigated in the Transition to eUHPLC
Pirkle-type (brush-type) chiral stationary phases (CSPs) are ideal candidates for the transition from enantioselective HPLC to enantioselective UHPLC (eUHPLC). The chiral selector is normally a small molecule that can be easily attached to smaller particles without modifying the synthetic process. Pirkle-type CSPs are less sensitive to phenomena such as clogging and excess of selector during their preparation, and, thanks to a more ordered structure, they are usually characterized by faster kinetics during the enantiorecognition process, when compared to polymer-based CSPs, such as the more widely used polysaccharide-based CSPs (6). Given the ease of access to sub-2-μm Pirkle-type materials and the easily scalable process to the desired particle size, these stationary phases provide not only a good starting point for the introduction of enantioselective UHPLC in current praxis, but also a good model to study the faster kinetics involved in a more exclusive type of interaction, represented by the chiral selector-more retained enantiomer system.
The first family of CSPs to be evaluated for the development of eHPLC columns was, in fact, the family of Pirkle-type CSPs (7). These CSPs were initially chosen as they provide a fast kinetic during the enantiorecognition process (essential for ultrafast separations), a good application field, and the necessary thermal and chemical inertness, ensuring compatibility with most mobile phases at high and ultrahigh pressure values. In 2010, our group developed the first CSP for eUHPLC, based on π-acidic bis-(3,5-dinitrobenzoyl)-derivative of trans-1,2-diaminocyclohexane (Figure 1), which was designed for HPLC in the 1980s and in the 1990s (8–10). Three phases grafted on 4.3-, 2.6-, and 1.9-μm silica particles were prepared by an improved, two-step synthetic procedure (11). The kinetic evaluation of the new materials packed in columns of different geometry confirmed the gain in efficiency that can be obtained by reducing the particle size, a general principle valid both in achiral and chiral chromatography (Figure 2). Furthermore, ultrafast separations (in the 15–40 seconds range) were obtained with the 1.9-μm CSP for six chiral test compounds (11).
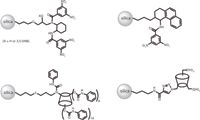
Figure 1: Structures of well-known CSPs investigated in the transition from eHPLC to eUHPLC. From left to right: DACH-DNB (11), Whelk-O1 (13-14), perphenylcarbamoylated β-cyclodextrin-CSP (15), and α-cyclodextrin immobilized via a triazole linkage (19).
During, the past two years, another Pirkle-type chiral selector, namely 4-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene (12) (see Figure 1), was successfully covalently immobilized in our laboratory onto 1.7-μm high-surface-area totally porous spherical silica particles (13). The resulting CSP was packed into columns of different geometries that yielded superior performances and ultrafast analyses. The resolution of a broad set of compounds, including alcohols, polar sulphoxides, phosphine oxides, and acidic drugs, was accomplished on the eUHPLC columns with analysis times as low as 30 s. After studying the kinetic performance of the eUHPLC columns from a theoretical point of view, we focused on the thermodynamics of the separation process, and a more in-depth comparison with 5-μm HPLC columns was made, in terms of speed gain, resolution, and solvent consumption (14).

Figure 2: (a) Van Deemter plots of DACH-DNB columns obtained in the first transition from eHPLC to eUHPLC and (b) two applications in the seconds time scale on the 1.9 μm UHPLC column. Column geometry: 50 mm à 4.1 mm. For experimental conditions, see reference 11.
Non Pirkle-Type Chiral Selectors Investigated in the Transition to eUHPLC
In 2010, sub-1-μm mesoporous silica particles functionalized with a perphenylcarbamoylated β-cyclodextrin (Figure 1) were described and tested in the rapid UHPLC enantioseparation of six basic and neutral racemates, achieving good resolution values (15). More recently, comparative HPLC separations of enantiomers with CSPs prepared by coating cellulose tris(4-chloro-3-methylphenylcarbamate) on totally porous and core–shell type silica particles with a nominal diameter of 2.6 μm were performed by Chankvetadze et al. (16).
Moreover, a sub-minute UHPLC enantioseparation of a folic acid precursor has been performed on a commercially available column packed with a 3-μm CSP based on cellulose tris(4-methylbenzoate) (17). Indeed, polysaccharide-based CSPs are the latest family of phases currently under investigation for the transition from eHPLC to eUHPLC: Cellulose and amylose tris(3,5-dimethylphenylcarbamate) derivatives have recently become available as 1.7-μm materials (18). In 2013, a click chemistry approach was successfully used to covalently immobilize a native α-cyclodextrin onto sub-2-μm spherical silica particles (19). The resulting CSP (see Figure 1) was packed into a short eUHPLC column that proved effective in the resolution of dansyl amino acids. Finally, it is worth mentioning the first application of heart-cutting two-dimensional ultrahigh-pressure liquid chromatography (2D–UHPLC) for monitoring asymmetric reactions in drug development (20). Reaction conversion and enantiomeric excess have been determined simultaneously for different reactions on different CSPs and mobile phase conditions.
Kinetic Evaluation of eUHPLC columns
The eUHPLC CSP based on 4-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene (1.7 μm particle size) was packed into columns of different geometry (13), that were then evaluated in terms of kinetic performance using van Deemter analysis, permeability data, and kinetic plots. In particular, a 100 mm × 4.6 mm eUHPLC column was chosen for evaluation against a commercially available eHPLC 250 mm × 4.6 mm, Whelk-O1 column (Regis Technologies). When choosing the column geometry for the eUHPLC column, a number of factors regarding the instrumentation had to be considered. In particular, both a 3.0-mm i.d. and a 4.6-mm i.d. column were used for the van Deemter analysis (13). However, given the fact that only a limited number of UHPLC instruments commercially available are fully compatible with organic solvents (including chlorinated ones) and that these normally display a high extra-column dispersion, the authors excluded 2.1 mm i.d. columns because a considerable loss in efficiency was observed (≥ 50 %) on currently available systems (compatible with chlorinated solvents). Instead, 3.0 mm and in particular 4.6 mm i.d. columns were used for the evaluation of kinetic data.
As reported in Figure 3 and in Table 1, our group was able to obtain efficiency results typically observed on achiral (C18) columns packed with 1.7 μm particles. A minimum height equivalent for a theoretical plate (HETPmin or Hmin) of 4.10 μm was observed at an interstitial optimal linear velocity (μinter,opt) of 5.22 mm/s (corresponding to a flow rate of approximately 2 mL/min), as shown in Figure 3(a), when performing the van Deemter analysis under normal-phase conditions (n-hexane/chloroform 90:10, v/v). The 100 mm × 4.6 mm i.d. eUHPLC column displayed theoretical plates up to approximately 250,000 N/m that corresponds to efficiency data typically obtained on non-enantioselective UHPLC columns packed with similar particles. Efficiency was even higher in reversed-phase mode, reaching a Hmin of 3.74 μm at a μinter,opt of 4.34 mm/s (corresponding to a flow rate of approximately 1.6 mL/min) and a N/m value of almost 280,000.

Figure 3: Comparison of kinetic performance (Van Deemter plots) obtained on the eHPLC Whelk-O1 (250 mm à 4.6 mm, 5 μm) and eUHPLC Whelk-O1 (100 mm à 4.6 mm, 1.7 μm) columns under (a) normal-phase and (b) reversed-phase conditions using naphthalene and ethylbenzene as probes. Normal-phase mobile phase: n-hexane/chloroform 90:10 (v/v); reversed-phase mobile phase: acetonitrile/water 60:40 (v/v). All analyses were performed at 25 °C. Data were not corrected for extra-column dispersion.
These results become more interesting when compared to the values observed with the eHPLC column, which represents the current standard column available in terms of geometry and particle size for chiral resolutions. In normal-phase mode, up to 73,000 N/m could be reached, corresponding to a Hmin of 13.71 μm at a μinter,opt of 2.77 mm/s (corresponding to a flow rate of 1.05 mL/min), while slightly better results were again obtained under reversed-phase conditions. It should be noted that for the construction of the van Deemter plots, small achiral probes were chosen.
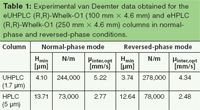
Table 1: Experimental van Deemter data obtained for the eUHPLC (R,R)-Whelk-O1 (100 mm à 4.6 mm) and eHPLC (R,R)-Whelk-O1 (250 mm à 4.6 mm) columns in normal-phase and reversed-phase conditions.
In a second paper on the eUHPLC columns, our group illustrated the efficiency of the supports when using chiral compounds as probes (14). A comparison between chiral and achiral probes was performed under normal-phase conditions using a mobile phase consisting of n-hexane/dichloromethane 80:20 (v/v) + 3% methanol. In particular, two chiral alcohols, namely acenaphthenol and benzoin, as well as a chiral sulphoxide, were used to investigate the van Deemter curves obtained on the eHPLC column (250 mm × 4.6 mm) and on the eUHPLC column (100 mm × 4.6 mm).
As shown in Figure 4, the diffusion of small achiral molecules can indeed be very different when compared to chiral ones. Although the optimum linear velocity was slightly lower for the chiral samples (μinter,opt of 4.24 mm/s) when compared to the achiral compounds such as 1,3-dinitrobenzene (μinter,opt of 5.07 mm/s), the efficiency values were quite similar, reaching values above 265,000 N/m and Hmin of 3.53 μm and 4.17 μm for 1,3-dinitrobenzene and acenaphthenol (second eluting peak), respectively.
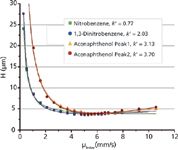
Figure 4: Comparison of van Deemter plots obtained on the eUHPLC Whelk-O1 column (100 mm à 4.6 mm, 1.7 μm) using both achiral (nitrobenzene and 1,3-dinitrobenzene) and chiral (acenaphthenol) molecules. Eluent: n-hexane/dichloromethane 80:20 (v/v) + 3% methanol; Temperature = 25 °C; UV detection at 254 nm. Data were not corrected for extra-column volume.
Similar results were obtained when analyzing chiral compounds such as benzoin (see Figure 5): The resolution of the two enantiomers of the chiral alcohol was achieved in less than 20 s at a linear velocity (μinter) of 15.79 mm/s, corresponding to a flow rate of 6 mL/min. Although the linear velocity was four times higher than the μinter,opt for the chiral sample, the corresponding loss in resolution was less than 40%. The results are fully compatible with the flat C term of the van Deemter curves observed with the column packed with 1.7 μm particles.
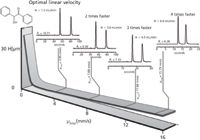
Figure 5: Chromatograms of benzoin obtained at increasing linear velocities (namely, μinter = 3.95, 7.89, 11.84, and 15.79 mm/s). Column: eUHPLC Whelk-O1, 1.7 μm (50 mm à 4.6 mm). Eluent: n-hexane/dichloromethane 80:20, v/v + 3% methanol; Temperature = 25° C; UV detection at 254 nm. Adapted and reproduced with permission from D. Kotoni, A. Ciogli, I. D’Acquarica, J. Kocergin, T. Szczerba, H. Ritchie, C. Villani, and F. Gasparrini, (2012), J. Chromatogr. A 1269, 226â241. © Elsevier.
Enantioselective columns packed with totally porous sub-2-μm particles showed an identical behaviour to conventional non-enantioselective columns, not only during the analysis of achiral samples, but also when using chiral compounds. The use of higher than optimal flow rates without significant efficiency loss is therefore possible on the eUHPLC column described and it performs as a UHPLC column under a variety of analytical conditions.
The fast kinetics of the enantioselective recognition process on Pirkle-type columns mean that when smaller particles are used, the gain obtained is not only a speed-related one but mostly an efficiency-correlated one. UHPLC is not (only) about fast separations, it is mainly about higher efficiency, which can be used either to reduce analysis time or to increment resolution. The advantages of the eUHPLC supports investigated are easy to recognize when observing the kinetic plots that compare the HPLC commercial column with the laboratory-made one (see Figure 6).

Figure 6: Kinetic plots showing a comparison of the different particle sizes under NP conditions. Eluent: n-hexane/chloroform 90:10 (v/v); η = 0.43 à 10â3 Pa s; Temperature = 25 °C; UV detection at 254 nm. (a) t0 vs. N plot; (b) L vs. N plot at 400 bar for the eHPLC column (5 μm; black curve, â²) and at 400 bar (orange curve, â¦) and 1000 bar (red curve, â) for the eUHPLC 1.7 μm column. Kinetic plots were prepared using linear velocities μ0 and H values of an unretained solute.
Kinetic plots are ideal to compare different columns as well as different analytical conditions (such as UHPLC versus HPLC or supercritical fluid chromatography (SFC), silica-based versus monolithic columns): This is becoming increasingly important in today's wide variety of instrumentation, methods, and support formats available in the market. Kinetic plots can be used as a geometry-independent comparative tool showing the potential efficiency of the different available solutions.
In eUHPLC, kinetic plots are important to better estimate the gain in terms of performance and analysis time obtained in the transition from eHPLC to eUHPLC. As shown in Figure 6, such plots can be used to quickly estimate which column offers the fastest separation for a given efficiency or the highest N value that can be obtained in a determined analysis time. The two kinetic curves in Figure 6 show the kinetic performance limit: A desired number of theoretical plates will be experimentally reached in the shortest time possible for the considered system, once the appropriate maximum system backpressure is chosen. The ΔPmax can be chosen on the basis of available instrumentation and typical operating conditions for each column. For example, a ΔPmax = 400 bar was used for the eHPLC column, considering the typical pressure under which HPLC columns are operated.
A maximum backpressure of 1000 bar was chosen for the eUHPLC column. By introducing an additional parameter (pressure), it is possible to compare columns based also on their permeability, which is linked, amongst other things, to the particle size. The use of the UHPLC column is justified up to 100,000 plates/column (for a ΔPmax of 1000 bar), while at higher efficiency values the B-term of the van Deemter equation predominates as the flow rate diminishes. For lower flow rates, the 5 μm HPLC column becomes more convenient (higher N value reached in less time). Theoretically, a higher number of N is possible on the 5 μm column, however this is at a considerable price: More than 100,000 N can be obtained using a 2-m-long column at very low flow rates, as demonstrated in Figure 6. Such performances, however, are not sustainable practically.
Ultrafast Resolutions of Chiral Test Solutes in eUHPLC
After studying in detail the kinetic performance of the eUHPLC columns, an evaluation of the thermodynamic properties of the columns in terms of retention, speed gain, and resolution was performed using more than 20 chiral samples, such as chiral alcohols, epoxides, sulphoxides, phosphine oxides, benzodiazepines and 2-aryloxypropionic acid esters (14). In all cases, the eUHPLC columns showed a consistent speed gain with respect to the HPLC columns. Analysis time was in some cases reduced by a 10-fold factor, while maintaining resolution at good values. In particular, ultrafast resolutions of enantiomers were obtained in the sub-minute time scale, as shown in Figure 7, for four racemates. In particular, an impressive enantioresolution of trans-stilbene oxide obtained with an analysis time of only 10 s, while many other compounds were successfully resolved in less than 25 s. A flow rate of 6 mL/min was used for the sub-minute eUHPLC applications without any column failure given by high pressure or high flow rate conditions. Finally, a 50 × 3.0 mm column was used to significantly reduce mobile phase consumption by a factor > 2, with limited extra-column band-broadening effects. This further proves that eUHPLC columns are convenient not only in terms of efficiency and speed gain, but also provide a more green option thanks to reduced solvent consumption.
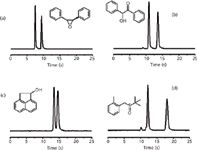
Figure 7: Ultrahigh speed resolution of racemates on the eUHPLC Whelk-O1 (50 mm à 4.6 mm) column. (a) trans-stilbene oxide; (b) benzoin; (c) acenaphthenol; (d) chiral sulphoxide. Eluent: n-hexane/dichloromethane 80:20, v/v + 3% methanol; Flow rate = 6.0 mL/min; Temperature = 25° C; UV detection at 254 nm.
Conclusion
Enantioselective UHPLC (eUHPLC) promises and delivers superior kinetic performances with respect to eHPLC in terms of high speed, efficiency, and resolution. Columns packed with brush-type CSPs, which were the first to be investigated in detail in the transition from eHPLC to eUHPLC, have been able to yield performances comparable to those achievable with non-enantioselective UHPLC columns. A similar transition has only marginally been observed with polysaccharide-based columns and probably a better understanding of the kinetics of the enantiomeric recognition as well as of the immobilization of the chiral selector on these CSPs is necessary to fully implement them in a sub-2-μm scenario. A second benefit in the use of eUHPLC columns is the reduced solvent consumption deriving from their smaller dimensions and the reduced analysis time. The possibility to directly use these columns in ultrahigh-pressure supercritical fluid chromatography enhances their environmentally friendly character, while widening their application field. Hopefully the growing academic achievements in the field will bring eUHPLC into the contemporary analytical laboratory.
Francesco Gasparrini is a full professor of organic chemistry at the Sapienza University of Rome, Italy. He was awarded for his research into the field "Structure Determination and Molecular Interactions" from the Italian Society of Chemistry in 2001 and in 2013 with the Arnaldo Liberti medal. In 2011, he received a gold medal in memory of Piero Pino. He is author of approximately 200 peer-reviewed papers in international scientific journals and 15 patents. Scientific achievements: Separation science (synthesis, characterization, and evaluation of stationary phases for UHPLC, HPLC, and SFC); study of stereochemically labile compounds by DUHPLC, DHPLC, and DNMR; elucidation of the enantioselective molecular recognition mechanism.
Ilaria D'Acquarica is an assistant professor of organic chemistry at the Sapienza University of Rome, Italy. In 1998 she received her PhD degree in pharmaceutical sciences. Her research interests lie in the field of separation chemistry, in particular in synthesis, characterization, and evaluation of novel chiral stationary phases for HPLC; HPLC analysis of complex biological samples; and enantioselective molecular recognition of resorc[4]arenes receptors in the gas-phase.
Dorina Kotoni completed her PhD in pharmaceutical sciences at the Sapienza University of Rome, Italy. Her research focused on the preparation and characterization of chiral stationary phases for enantioselective UHPLC and UHPSFC, as well as on the development of new HILIC materials for rapid speed analysis of carbohydrates. She is now a principal scientist at Novartis Pharma AG, working with a focus on the transition from HPLC to fast and ultra-fast liquid chromatography and on the analysis of UV inactive compounds.
Alessia Ciogli is an assistant professor of organic chemistry at the Sapienza University of Rome, Italy. In 2006 she received her PhD degree in pharmaceutical sciences and in 2008 she was a research fellow at the University of Wien with Prof. Wolfgang Lindner. She has been involved in a number of academic research projects, mainly focusing on the synthesis of solid supports for chromatographic applications and the stereochemical investigation of chiral molecules, especially of stereochemically labile compounds.
Marco Pierini is an associate professor of organic chemistry at the Sapienza University of Rome, Italy. Pierini's research interests include: Molecular recognition of chiral species by theoretical and experimental approaches; simulation of supramolecular adducts by molecular docking procedures; determination of kinetics and thermodynamic parameters involved in conformational and configurational changes of chiral molecules by DHPLC and spectroscopic techniques; studies of solvent effects based on classical and LSER approaches; and development of original computer software oriented to the rationalization of experimental data.
Jelena Kocergin received her BS in biochemistry from the University of Missouri, USA, in 2001, and joined Regis Technologies, Inc. in 2002 in a sales development position. She became an international sales manager in 2005 and become Director for the Chromatography and Separations Department in 2009. She works in global business, sales, and product development position for Regis' chromatography products and services.
Harald Ritchie is currently the European Operations Director of Trajan Scientific and Medical. Prior to this role he was the Technology and Business Development Director at Thermo Fisher Scientific's Chromatography and Mass Spectrometry Consumables business. His experience in the design and manufacture of chromatographic stationary phases has been developed over the last 30 years in the industry.
Claudio Villani is a full professor of organic chemistry since 2006 at the Sapienza University of Rome, Italy. His interests lie in the field of organic chemistry, with an emphasis on the application of chromatography and spectroscopy (NMR, CD, MS) to problems in static and dynamic stereochemistry. He is a member of the editorial board of Chirality and, since 2010, of the Permanent International Scientific Committee of the Chirality series of symposia.
References
(1) A. de Villiers, F. Lestremau, R. Szucs, S. Gélébart, F. David, and P. Sandra, J. Chromatogr. A 1127, 60–69 (2006).
(2) D.T.-T. Nguyen, D. Guillarme, S. Rudaz, and J.-L. Veuthey, J. Chromatogr. A 1128, 105–113 (2006).
(3) D. Guillarme, D.T.-T. Nguyen, S. Rudaz, and J.-L. Veuthey, J. Chromatogr. A 1149, 20–29 (2007).
(4) J.R. Mazzeo, U.D. Neue, M. Kele, and R.S. Pumb, Anal. Chem. 77, 460A–467A (2005).
(5) Y. Wang, F. Ai, S.-C. Ng, and T.T.Y. Tan, J. Chromatogr. A 1228, 99–109 (2012).
(6) F. Gritti and G. Guiochon, J. Chromatogr. A 1332, 35–45 (2014).
(7) W.H. Pirkle, J.M. Finn, J.L. Schreiner, and B.C. Hamper, J. Am. Chem. Soc. 103, 3964–3966 (1981).
(8) (a) F. Gasparrini, F. La Torre, D. Misiti, and C. Villani, J. Chromatogr. 539, 25–36 (1991); (b) F. Gasparrini, D. Misiti, and C. Villani, Chirality 4, 447–458 (1992).
(9) F. Gasparrini, D. Misiti, and C. Villani, J. Chromatogr. A 906, 35–50 (2001).
(10) G. Cancelliere, I. D'Acquarica, F. Gasparrini, M. Maggini, D. Misiti, and C. Villani, J.Sep. Sci. 29, 770–781 (2006).
(11) G. Cancelliere, A. Ciogli, I. D'Acquarica, F. Gasparrini, J. Kocergin, D. Misiti, M. Pierini, H. Ritchie, P. Simone, and C. Villani, J. Chromatogr. A 1217, 990–999 (2010).
(12) (a) W.H. Pirkle, C.J. Welch, and B. Lamm, J. Org. Chem. 57, 3854–3860 (1992); (b) W.H. Pirkle and C.J. Welch, J. Liq. Chromatogr. 15, 1947–1955 (1992).
(13) D. Kotoni, A. Ciogli, C. Molinaro, I. D'Acquarica, J. Kocergin, T. Szczerba, H. Ritchie, C. Villani, and F. Gasparrini, Anal. Chem. 84, 6805-6813 (2012).
(14) D. Kotoni, A. Ciogli, I. D'Acquarica, J. Kocergin, T. Szczerba, H. Ritchie, C. Villani, and F. Gasparrini, J. Chromatogr. A 1269, 226-241 (2012).
(15) F. Ai, L. Li, S.-C. Ng, and T.T.Y. Tan, J. Chromatogr. A 1217, 7502–7506 (2010).
(16) K. Lomsadze, G. Jibuti, T. Farkas, and B. Chankvetadze, J. Chromatogr. A 1234, 50–55 (2012).
(17) D. Frühauf and M. Juza, J. Chromatogr. A 1269, 242-254 (2012).
(18) (a) D.W. House and A.A. Oroskar, U.S. patent application publ., US 20130277304, 2013; (b) C. Hamman, M. Wong, I. Aliagas, D.F. Ortwine, J. Pease, D.E. Schmidt Jr., and J. Victorino, J. Chromatogr. A 1305, 310–319 (2013).
(19) F. Ai, Y. Wang, H. Chen, Y. Yang, T.T.Y. Tan, and S.-C. Ng, Analyst 138, 2289–2294 (2013).
(20) S. Ma, N. Grinberg, N. Haddad, S. Rodriguez, et al., Org. Process Res. Dev. 17, 806–810 (2013).

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.












