Evaluating the Temperature Shift in Analytical Temperature Rising Elution Fractionation
LCGC North America
A new method is presented to evaluate the temperature shift observed in analytical temperature rising elution fractionation (ATREF).
This article presents a new method to evaluate the temperature shift observed in analytical temperature rising elution fractionation (ATREF). The evaluation is based on the dependence of the measured peak temperature as a function of heating rates. Application of the proposed method does not require any knowledge of the fluid circuit characteristics geometry and avoids the use of narrow preparative TREF standards. The results are found to be more accurate than the method that is usually applied.
Since the publication of the Wild article (1) in 1982, analytical temperature rising elution fractionation (ATREF) has been a method of choice for polyolefin characterization. In 1991 Exxon introduced the first patents in which polyethylenes were described by their compositional distribution breadth index (CDBI), an ATREF parameter equivalent to the polydispersity index of the molecular weight distribution. Exxon was followed by other material producers like Dow and Cryovac, and the number of patents incorporating claims covered by ATREF analysis steadily increased to about 30 patents per year. At the beginning, the ATREF systems were built by modifying gel permeation chromatography (GPC) systems. The increasing demand for ATREF measurements fostered the introduction of the first automated commercial ATREF system by Polymer Char in 1996. No standardized method was published for this technique, and most of the patents make reference to Wild's publication (1).
To calculate the CDBI, several standards of polyethylene with narrow compositions and known short-chain branching characteristics are injected into the ATREF unit and chromatograms are recorded as a function of time while monitoring the column temperature. These data are used to construct thermograms, in which the detector signal is plotted as a function of the column temperature. The recorded column temperature is usually higher than the real melting temperature of the eluted fraction because of the delay between melting in the column and detection. This delay depends on several experimental parameters, including column dimensions, tubing characteristics between the column and detector, and heating and elution rates. These equipment-specific parameters prevent the use of any short chain branches (SCB)-elution temperature calibration curves available in the literature (1–3). Actually, as recommended by the commercial ATREF manufacturer (4), the use of an identical procedure and identical equipment is mandatory to be able to compare samples.
The procedure would be greatly simplified with a robust method to calculate the temperature shift, allowing for the correlation between results obtained with different instruments and methods.
To circumvent that problem, the temperature shift can be calculated based on the tubing volume between the column and the detector. As the heating rate and the flow rate during the heating step are usually constants, the tubing volume can be converted into temperature shift using the following relationship:
temperature shift (°C) = heating rate (°C/min) × tubing volume (mL)/flow rate (mL/min) [1]
As this method assumes a detailed knowledge of the tubing and does not take into consideration the heat transfer delay in the column heating system, it is expected that the calculated temperature shift will be lower than the actual one.
An alternative method to evaluate the temperature shift is to analyze narrow preparative TREF standards (5,6). In this case, the temperature shift is given by the difference between the measured ATREF peak temperature and the preparative TREF temperature of the standard; the difficulty of the method is the fact that narrow TREF standards, isothermally eluted in a temperature interval of about 3 °C, are not easily available. Additionally, the high preparative column volume introduces measurement errors close to the temperature interval used to elute the preparative fraction (3 °C). Another potential issue with this method is the fact that usually the analytical TREF solvent (trichlorobenzene) is different from the solvent used in preparative TREF (xylene).
In this article, we present a new method to evaluate the temperature shift for ATREF that does not require either the detailed knowledge of the tubing between the column and the detector or the availability of narrow standards obtained by preparative TREF. To apply the method, it is possible to use any unimodal sample such as a commercial metallocene polyethylene or high-density polyethylene. The method is based on the idea that the temperature shift is zero for isothermal elution steps. Because finite heating rates are used with ATREF, we propose to find the real melting temperature by analyzing the same sample with different heating rates and to extrapolate the measured elution temperatures to isothermal conditions. After finding the real melting temperature by extrapolation, we can then use a general rule to calculate the temperature shift for a given heating rate.
Experimental
ATREF apparatus
The analyses were done using a TREF-Crystaf model 300 instrument (Polymer Char). The TREF unit is integrated into an Agilent Technologies model 7890 GC system oven, which is kept as originally supplied by the manufacturer with the exception that the main switch also activates the TREF dedicated power supply and all other electronics. TREF control software is used to set the oven temperature program through one of the computer serial ports. The elution step was performed with an Intelligent Pump Series 300 (IP300, Flom Corporation), located close to the oven and controlled by the TREF software. The concentrations of the eluted polymer fractions were measured with the built-in infrared detector, using wavelengths between 2800 and 3000 cm-1.
Samples
Four commercial metallocene polyethylenes were analyzed using the ATREF system. The selected samples have narrow short-chain branching distributions and the following densities: 0.923, 0.934, 0.947, and 0.955 g/cm3.
ATREF Methods
Each sample was dissolved in 1,2,4-trichlorobenzene (TCB Spectropure dry, Biosolve Chimie, CAS 120-82-1) at 150 °C to obtain a 2 mg/mL solution. The solvent was stabilized with 0.3% of antioxidant (butylated hydroxytoluene, BHT, CAS 128-37-0). About 300 µL of the hot solution was injected at 150 °C in the ATREF column (100 mm × 0.25 in.) filled with metallic shots provided by Polymer Char. The column filled with solution was fast cooled (1 °C/min) to 100 °C, and then was further cooled to 30 °C with a cooling rate of 0.1 °C/min. After the cooling step, the polymer was eluted from the column, using a flow rate of 0.5 mL/min. Before starting the heating to 130 °C, the column was maintained at 30 °C for 30 min. For each sample, four different heating rates were used: 0.2, 0.5, 1.0, and 2.0 °C/min.
Results and Discussion
Figure 1 is an overlay of the four elution profiles (trefograms) recorded for the 0.947-g/cm3 density polyethylene. The total elution step increases from 80 min for a heating rate of 2 °C/min to 530 min for a heating rate of 0.2 °C/min. Trefograms of the other three standards showed similar behavior.
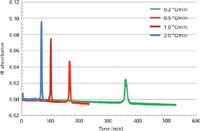
Figure 1: Overlaid chromatograms (infrared detector signal as a function of time) recorded using different heating rates for the 0.947-g/cm3 polyethylene.
Figure 2 shows the same elution profiles as a function of the recorded temperature during the elution step. The overlay of the four thermograms is representative of the results. One can note that the peak temperature and the peak width increase with the heating rate.
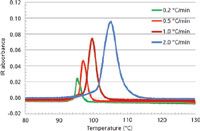
Figure 2: Overlaid thermograms (infrared detector signal as a function of recorded temperature) for the 0.947-g/cm3 polyethylene.
The measured temperatures for each experiment were automatically corrected by the commercial TREF software based on the tubing lines between the TREF column and detector cell, and the elution rate. The results are given in Table I. The differences between the peak temperatures obtained with 0.2 and 2.0 °C/min are about 3 °C.
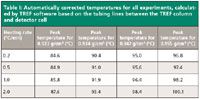
Table I: Automatically corrected temperatures for all experiments, calculated by TREF software based on the tubing lines between the TREF column and detector cell
In Figure 3 the measured peak temperatures are plotted as a function of the heating rates for the different polyethylenes used in this work. The extrapolated elution temperatures at zero heating rates represent the real melting temperatures. The obtained parallel lines of the linear dependence of the elution temperatures vs. heating rates for the different densities implies the existence of a unique parameter to calculate the temperature shifts. For a flow rate of 0.5 mL/min, the observed average slope of 5.1 min corresponds to the time needed for a polymer fraction to be melted and travel to the detector. This time delay takes into account the tubing volume as well as other parameters such as the heat transfer between the oven and the column, and the necessary time to solubilize the melted polymer.
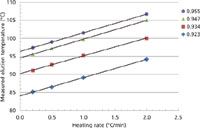
Figure 3: Plot of measured peak temperatures as a function of heating rates for four metallocene polyethylenes.
With this parameter, the temperature shift can be calculated for any heating rate with the following relationship:
temperature shift (°C) = heating rate (°C/min) × time delay (min) [2]
With this equation, the measured temperatures for the 16 ATREF experiments were corrected; the results are presented in Table II. A maximum difference of 0.4 °C was noted, which is about 10% of the error recorded with the previous method. This difference is close to the variability of the ATREF method when determining the peak temperature by repeated measurements (for six measurements using a heating rate of 1 °C/min done on HDPE NIST 1475, the average uncorrected elution temperature is 101.3 °C, and the standard deviation is 0.2 °C).
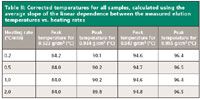
Table II: Corrected temperatures for all samples, calculated using the average slope of the linear dependence between the measured elution temperatures vs. heating rates
Conclusion
A new and simple method is proposed that allows for evaluating the temperature shift in analytical temperature rising elution fractionation (ATREF). This technique is based on the variation of the measured peak temperature as a function of the heating rate during the elution step and does not require the detailed knowledge of the instrument tubing or the availability of standards obtained by preparative TREF. To apply the method, it is possible to use any polyolefin sample, as long as it has a narrow composition distribution.
Apart from measuring the correct melting temperature with higher precision, the method opens the possibility of creating "universal" calibration curves (SCB vs. temperature) for each type of comonomer. Such a procedure would improve the interlaboratory reproducibility for the calculation of CDBI and other parameters characterizing the chemical composition distribution of different types of polyethylenes.
The method was initially developed on a commercial ATREF system, but should be valid with in-house ATREF instruments with different configurations.
References
L. Wild, T.R. Ryle, D.C. Knobeloch, and I.R. Peat, J. Polym. Sci.: Polym. Phys. Ed. 20, 441–455 (1982).
F.M. Mirabella, J. Polym. Sci: Part B: Polymer Physics 39, 2819–2832 (2001).
F. Chen, R.A. Shanks, and G. Amarasinghe, Polymer 42, 4579–4587 (2001).
Polymer Char TREF User Manual, page 2–17 ( 2011).
A.G. Boborodea, D. Daoust, A. Jonas, and C. Bailly, LCGC North Amer. 22(1), 52–57 (2004).
US 7389678, A.G. Boborodea, C. Bailly, D. Daoust, A. Jonas, and B. Nysten, Column for analytical temperature rising elution fractionation (ATREF).
Adrian Boborodea and André Luciani are with Certech ASBL in Seneffe, Belgium. Direct correspondence to:

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)