The Essentials: Troubleshooting Real HPLC Problems
An excerpt from LCGC's e-learning tutorial on HPLC problems at CHROMacademy.com.
An excerpt from LCGC's e-learning tutorial on HPLC problems at
Photo Credit: DrAfter123/Getty Images

The following questions were submitted by CHROMacademy members as part of our "HPLC Troubleshooting Master Class" series, which focused on problems in the modern chromatography laboratory.
Can reversing a modern reversed-phase HPLC column really damage it?
Columns are packed using a constant-pressure rig with a solvent packing slurry forced through the column blank at higher pressure than the intended application limit, with the outlet frit and fitting in place, but the inlet end directly connected to the packing rig reservoir. Although manufacturers do a great job in ensuring the packing is homogenous from inlet to outlet, there is always the possibility the material at the outlet is more densely packed, and so to avoid settling of the packed bed and a void formation, one should observe the "directionality" of the column.
When column efficiency declines or peaks begin to split or shoulder, users sometimes wish to reverse the column direction to flush strongly adsorbed material from the inlet frit or packing material at the "front" of the column. This is possible using modern high performance liquid chromatography (HPLC) columns without too much risk, other than the packed bed "settling" in the reversed direction - resulting in the same problems all over again. Remember to disconnect your detector from the column when flushing to avoid contamination.
If you inject a large-volume sample that is different from the starting mobile phase in a gradient can you get partitioning of analyte between the injection slug and the mobile phase?
Yes! As the slug of analyte moves towards the column it will begin to partition at the eluent or diluent interface; however, this mixing is often incomplete, especially because the distance and volume between the injector and column in modern HPLC systems is so small. At the head of the column, especially in gradient analysis, all analytes come to a standstill. Those analyte molecules associated with the most highly eluting solvent (usually, and incorrectly, the sample diluent) will partition down the bed somewhat, giving two "discrete" regions of analyte at the column head - those associated mainly with eluent and those associated mainly with the diluent as they entered the column - leading to a broad and often misshapen peak.
One should match both the eluotropic and ionic strength of the sample diluent with the initial mobile-phase composition. Where this is not possible (typically because of solubility issues), one should use the lowest injection volume possible to allow effective mixing before, and at the head of, the column, which will help to mitigate some of the effects mentioned above. If solubility is an issue, use a suitable solvent to dissolve the sample initially, but then reduce the amount of organic solvent in any subsequent dilution steps.
I'm having problems transferring a method between a binary and quaternary ultrahigh-pressure liquid chromatography (UHPLC) system. I've calculated and adjusted for the difference in gradient dwell time but still cannot reproduce the chromatography.
In this problem, even though the system dwell volumes have been catered for by altering the gradient start time (either starting the gradient before injection or inserting an isocratic hold at the start of the gradient), the separation selectivity still cannot be reproduced. This is a problem that we are seeing more frequently, and it stems from the differences in the way the gradients are formed and delivered and the inherent pump volumes associated with solvent mixing. It is important to note that we have also seen this problem when transferring methods between different quaternary HPLC systems - so the issue is not only associated with method transfer between high- and low-pressure mixing systems.
UHPLC systems have markedly different technologies to mix the required gradient composition on-line and, as well as having different gradient composition delay characteristics, there will be differences in the "slope and wash out" characteristics of the gradient delivery profiles as shown in Figure 1.
Figure 1: Gradient profile determination for two UHPLC systems.
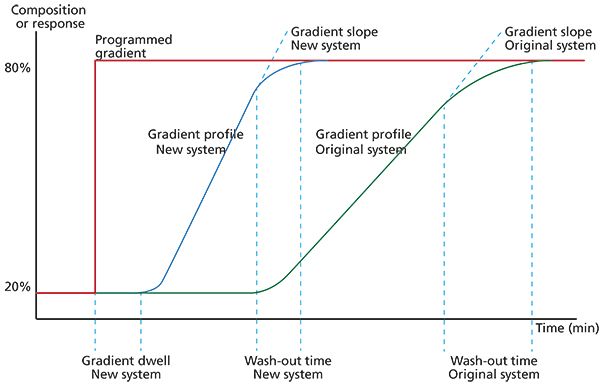
Unless one can compensate for the differences in the gradient delivery characteristics, than successful method translation may be difficult. Although some UHPLC systems are equipped with software that can simulate differences from certain manufacturers equipment, not all systems are similarly equipped.
While not ideal, we have recently adopted the following approximation to adjust the gradient profile between systems, after the gradient slope or wash out volume (washout time × flow rate) has been characterized: New segment time = Original segment time × (Original system washout volume/New system washout volume).
Adjusting the gradient segment times as outlined above will adjust the slope of the gradient delivered by the new system. When combined with a similar adjustment to account for dwell volume differences at the beginning of the analysis, this approach can help to more closely match the solvent composition at the head of the analytical column at any given time within the gradient.
Get the full tutorial at www.chromacademy.com/Essentials (Free until 20 July 2014).
This article is from The Column. The full issue can be found here: http://www.chromatographyonline.com/vol-10-no-12-column-july-07-2014-europe-and-asia-pdf

Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
The Effect of Time and Tide On PFAS Concentrations in Estuaries
April 8th 2025Oliver Jones and Navneet Singh from RMIT University, Melbourne, Australia discuss a recent study they conducted to investigate the relationship between tidal cycles and PFAS concentrations in estuarine systems, and offer practical advice on the sample preparation and LC–MS/MS techniques they used to achieve the best results.










