Enhancing Signal-to-Noise
LCGC North America
In order to improve LLOQ or LOD, or to increase precision in any case where S/N degrades precision, an increase in S/N will be beneficial. Here, John Dolan focuses on improvements in signal-to-noise.
The signal-to-noise ratio (S/N) in a liquid chromatography (LC) separation usually is defined as shown in Figure 1. The noise is measured between two lines bracketing the baseline and the signal is measured from the middle of the baseline to the top of the peak. S/N is merely the signal divided by the noise. There are some other ways to determine S/N, but that is not the point of the current discussion. My comments here will apply no matter what technique you use for measurement and calculation of S/N.

John W. Dolan
As was discussed in an earlier installment of "LC Troubleshooting" (1), S/N is of most concern when it makes a significant contribution to the error of chromatographic measurements. This usually occurs for S/N << 100, and is of most concern when we approach the lower limit of quantification (LLOQ) and limit of detection (LOD). Once again, it doesn't matter how we define LLOQ or LOD, the principles presented here apply in all cases.
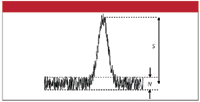
Figure 1: Measurement of the signal and noise from a chromatogram.
Although peak area measurements are used most commonly for quantitative purposes with large peaks, peak height is more important than peak area when peaks approaching the lower method limits are concerned. This is because both S and N in the S/N calculation are based upon height, not area. To improve the LLOQ or LOD, or to increase precision in any case in which S/N degrades precision, an increase in S/N will be beneficial. This can be done by either increasing the signal, decreasing the noise, or both. This month's "LC Troubleshooting" will focus on improvements in S/N.
Improving the Signal
Of course, anything that will make the peak taller results in an increase in signal. There are several ways to increase the response for the analyte; some of these are listed in the sidebar.
If you are using a UV detector, it may be possible to choose a better wavelength than the one currently in use. If the analyte has a UV maximum that is >220 nm, usually it is beneficial to use that wavelength for detection because it improves selectivity — the ability to quantitatively measure one compound in the presence of others. However, the best wavelength for selectivity might not be the best for the detector response. Almost all organic compounds have a strong "end absorbance" near 200 nm. The response in this region might be much larger than at the UV maximum at higher wavelengths, so a reduction of the detection wavelength to <220 nm often will increase the response.
Not all UV detectors are created equal, so it might be possible to gain some response by changing to a better detector. This might be a newer detector or one of a different design. Also, a detector operating on a different principle can give a better response than the one that you are using. For example, evaporative light scattering detection (ELSD) often is at least an order of magnitude more sensitive than refractive index (RI) detection.
Most of us avoid any kind of derivatization of our sample if we can avoid it because of the inconvenience, expense, and possible introduction of additional error. However, if you modify the analyte, it might be possible to improve the detector response. For example, if you can make a fluorescent derivative of your compound, you might be able to switch from UV to fluorescence detection and improve both the sensitivity and selectivity of the method.
One of the simplest ways to increase the response for an analyte is to inject more sample. It is very seldom that we inject all or nearly all of the sample. In many cases, the method will accommodate larger injections, especially if the injection conditions are modified somewhat. For example, if sample preparation can be adjusted to reduce sample dilution, you might be able to inject a larger mass of sample in the same volume. Alternatively, if you use an injection solvent that is more dilute than the mobile phase, you might be able to inject very large sample volumes — 100 μL or more in some cases.
Because peak height is the critical factor when determining the signal, anything we can do to shift more of the peak area into the height will be beneficial. To reduce the peak width, the peak volume has to be decreased; reducing the peak width by increasing the flow rate usually will have no advantage because it does not change the peak volume.
When we develop a new LC method, we usually like the retention factor k to be in the region of 1 < k < 20 to get "good chromatography." This also keeps the first peak away from the garbage peak that usually appears at the beginning of the chromatogram. However, a smaller k-value will mean shorter retention times plus narrower and taller peaks. When you make a change in retention, you have to be sure the peak of interest does not have new interference problems.
The peak volume is related to the column volume, so a smaller column also will reduce the peak volume and increase the peak height proportionally. A simple way to reduce the column volume is to reduce the column diameter and hold the other column properties the same. For example, you can replace a 150 mm × 4.6 mm column with a 150 mm × 2.1 mm one. You'll also have to reduce the flow rate to keep the linear velocity the same. Just lower the flow rate in proportion to the reduction in cross-sectional area: (4.6 /2.1)2 ≈ 5, so a 1.0 mL/min flow rate would be reduced to 0.2 mL/min. In theory, this should reduce the peak volume fivefold, so the peak height would increase by the same amount. Similarly, a shorter column will reduce the column volume and thus the peak volume in proportion. If you change the column volume, you need to be sure the sample does not overload the new column configuration, or you may need to reduce the sample mass on column, which will mean that you won't get the full gain in signal from a smaller column.
Another way to reduce the peak width is to use a column with a higher plate number. This is done most easily by a reduction of the packing particle size. For example, the column packing can be changed from a 5-μm to a 3-μm particle diameter. This also will cause the pressure to increase if the same column dimensions and flow rate are used, but the peaks will be narrower and taller, thus enhancing the signal.
Decreasing the Noise
Just as an increase in the signal will improve S/N, a decrease in the baseline noise will provide a similar benefit. One of the simplest ways to reduce baseline noise is to increase the detector time constant. The time constant is an electronic filter that is part of all LC detectors. Various types of filters are available ranging from a simple resistance-capacitance (RC) filter to a more elaborate electronic noise filter. The actions of all these filters are the same — they average the signal over a certain period of time and report the average instead of individual values. As long as the averaging time is small in relation to the peak width, the noise will be reduced, but no degradation of the signal will be seen. If the time constant is sufficiently large, it will average too many points together and can decrease the peak height ("clip" the peak apex) and also reduce resolution between partially resolved peaks. Filters usually are described by their time constant in seconds; this is sometimes referred to as the rise time or response time. A good rule of thumb is to set the time constant to ≈1/10 the peak width of the narrowest peak of interest. So, for example, if the first peak of interest is 10-s wide at the baseline between tangents drawn to the sides of the peak, a time constant of 1 s is appropriate. The default value for different detectors is likely to be different, so just accepting the default might not give you the best filtering for your situation. For example, I just looked in the manual for one UV detector and it has time constants of 0.05–10.0 s available, yet the noise specification is stated with a 1-s time constant. You'll have to check your detector to see what is available and adjust the time constant according to your chromatograms.

A similar, but distinct, setting in the chromatographic data system is used to combine data points. You often can reduce the noise by increasing signal bunching. Whereas the time constant, or filter, averages the signal over a particular time, bunching combines several data points and reports the sum. This has a very similar result to the time constant, but because they both modify the data using different techniques, it is worthwhile looking at both settings to see which one is more advantageous or if a combined change is best. The bunch rate or data slice rate is the usual unit of measure for signal bunching. As a general rule, the data rate should be set to be ≈20 points across the peak. So the 10-s peak width mentioned earlier should have a data rate of 2 Hz, or one (combined) point every 0.5 s. You also should be aware that there are two different types of data rates in a data system, sometimes called by different names. One relates to how the raw data are collected. This usually is fairly fast, perhaps 50–100 Hz. The second controls how the data are processed for reporting; this is the slower bunch rate that is our focus. You can take a raw data file and try several different bunch rates on it to see the results and it does not damage the original raw data. However, if you make the original data collection rate too slow, you will not be able to evaluate the effect of a faster data rate without collecting more data.
The effect of column temperature most commonly is considered in terms of changes in retention time. However, because optical detectors, such as UV, are susceptible to refractive index effects, a change in detector temperature can affect baseline noise as well. Usually, operation of the column in a column oven and keeping the instrument away from direct air currents is sufficient, but sometimes better temperature control is a good idea. Always operate the column in a column oven, even if it is near ambient temperature. Look up — is there an HVAC vent above the instrument that blows hot or cold air on the instrument? Even though the laboratory as a whole might have a constant temperature, the microenvironment around the LC system might not be quite so stable. Blocking or redirecting a heating vent sometimes can help.
When operating near the method limits, variables that are of little consequence for high-concentration methods can be critical. Be sure to use the best reagents possible. High performance liquid chromatography (HPLC)-grade solvents and laboratory-prepared HPLC-grade water are standard in the industry, but sometimes workers tend to use less pure solvents to save money with isocratic runs, particularly when S/N is not important. Similarly, buffers, salts, and other mobile phase additives come in different reagent qualities. Use better reagent purity and you may observe quieter baselines.
On-line mixing is a standard way to prepare mobile phases for LC methods. However, there is always a compromise between the quality or completeness of mixing and the volume of the mixer. As a result, most LC systems are designed for as small a gradient dwell volume as possible while providing adequate mixing. Increasing the mixer volume can provide better mixing and reduce the baseline noise. Some systems have a choice of mixer sizes from which to choose. For example, one system I have used has several mixing chambers that can be connected in series to provide mixing volumes from ≈1 mL to ≈5 mL. Another system has a 400-μL auxiliary mixer that could be added for improved mixing. Another trick that you can use to improve on-line mixing is to partially mix the two mobile phase components. For example, mix 5% of the A-solvent in B and 5% of the B-solvent in A, and adjust the program settings accordingly. This partially pre-mixed mobile phase will blend together better than the pure solvents alone. And there are some setups, such as when RI detection is used, where on-line mixing never is good enough. No on-line mixer can do as well as a hand-mixed mobile phase.
Today's pumps operate very reliably, but in some cases, it is possible to reduce pump pulsations and reduce the baseline noise. Sometimes pump pulsations are due to an imbalance or worn part in a pump, such as a check valve or piston seal that leaks. If you are in doubt, perform a routine maintenance session on the pump.
Conclusions
When operating near the lower limits of an LC method, S/N can be the limiting factor in method performance. When this is the case, you will need to address the problem by increasing the signal, reducing the noise, or both. We have looked at some of the possible ways to accomplish this. Some changes are very simple and can be done after the data are gathered. Others may require alterations of the LC system. I recommend following the "easy over powerful" strategy: make the simple adjustments first and see if they are adequate, then you can attack the harder ones only if really necessary.
John W. Dolan
"LC Troubleshooting" Editor John W. Dolan is Vice-President of LC Resources, Walnut Creek, California; and a member of LCGC's editorial advisory board. Direct correspondence about this column to "LC Troubleshooting," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail John.Dolan@LCResources.com.
For an ongoing discussion of LC troubleshooting with John Dolan and other chromatographers, visit the Chromatography Forum discussion group at http://www.chromforum.org.
References
(1) J.W. Dolan, LCGC 27(4), 306–312 (2009).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







