Enhanced-Fluidity Liquid Chromatography: Connecting the Dots Between Supercritical Fluid Chromatography, Conventional Subcritical Fluid Chromatography, and HPLC
Special Issues
Enhanced-fluidity liquids are organic solvents or organic–aqueous solvents mixed with high proportions of liquefied gases, such as carbon dioxide.
Enhanced-fluidity liquids are organic solvents or organic–aqueous solvents mixed with high proportions of liquefied gases, such as carbon dioxide. These subcritical solvents share the positive attributes of supercritical fluids (fast diffusion rates and low viscosities) and the positive attributes of commonly used liquids (high solvent strength). These solvent properties provide enhanced efficiency for reversed-phase, conventional normal-phase, hydrophilic-interaction, and size-exclusion chromatography. The capabilities of enhanced-fluidity liquid chromatography for highly polar compounds is described and the value of using the entire continuum of 0–100% carbon dioxide solvent systems is discussed in terms of changes in mobile-phase properties and applications.
Enhanced-fluidity liquids (EFL) are mixtures of conventional liquids to which dissolved gases, such as carbon dioxide, are added. Our group coined this term in 1991 to explain the fact that these solvents possess fluidity (inverse of viscosity) that is markedly higher than typical liquids (1). This was at a time when supercritical fluid chromatography (SFC) was gaining interest. These mixtures are described as gas expanded liquids (GXLs) in the chemical engineering literature (2), but both terms are merely descriptive of the physical phenomena involved in producing the mixture. The addition of a liquefied gas to a conventional liquid causes considerable volume expansion of the mixture, and the fluidity of mixture increases substantially. Today, much of the work in SFC is actually performed under subcritical fluid conditions, meaning the separations are being performed below the critical point of the solvent. Enhanced-fluidity liquids are indeed subcritical solvents, but enhanced-fluidity liquid chromatography (EFLC) uses a smaller proportion of liquefied gas in the mobile phase than in typical subcritical fluid chromatography, which is 0–50% organic modifier. In EFLC, conditions from 100% to 50% conventional liquid combined with a liquefied gas are used in the mixtures. Therefore, by combining conventional subcritical fluid chromatography with EFLC, the entire solvent range of 0–100% organic solvent is spanned. This solvent range is highly useful for chromatographic applications. Like in SFC, EFLC typically uses carbon dioxide as the liquefied gas, but other liquefied gases have also been evaluated, such as fluoroform (3,4).
Optimization of Chromatography
In high performance liquid chromatography (HPLC), the fastest separations are achieved when working at the highest possible pressure of the chromatographic instrument. Using reduced (dimensionless) plate height, h, and reduced velocity, v, as defined in equations 1 and 2, the Knox-Saleem equation (equation 3) illustrates a linear relationship between retention separation time, to, and viscosity,
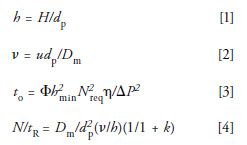
Click here to view full-size graphic
where H is plate height, u is linear velocity, dp is the particle size of the packing, Dm is the diffusion coefficient of the analyte, Nreq is the required chromatographic efficiency for a given separation, to is the retention time (seconds), Φ is the dimensionless flow resistance parameter, η is the viscosity of the mobile phase, ΔP is the pressure drop across the column, and tR is the retention time (5). Equation 3 assumes an optimized chromatographic system in terms of column length, particle size, and flow rate. Assuming maintenance of operating conditions near these conditions, a substantial decrease in time of analysis is observed by lowering the viscosity of the mobile phase. Finally, Guiochon and others illustrated that the separation power, N/tR (6,7) can be increased by increasing the mobile-phase diffusion coefficient assuming the other parameters do not vary much and the entire separation is functioning at the optimum reduced velocity where k is the retention factor.
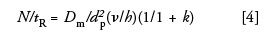
Click here to view full-size graphic
Properties of EFL Mixtures
Solvent Strength and Dielectric Constant
An interesting attribute of enhanced-fluidity liquid mixtures is that as much as 60 wt% liquefied gas can be added before considerable loss of solvent strength occurs (1,4,8). Mixtures of alcohols and carbon dioxide are robust in their solvent strength, particularly methanol–carbon dioxide mixtures. Aida and Inomato (9) studied the molecular structure of methanol-carbon dioxide mixtures using molecular dynamics (MD) simulations. Their study illustrated that up to 50 wt% carbon dioxide can be added to methanol before impacting the hydrogen bond (H-bond) network. However, below that percentage the H-bond network degraded. Their data and the solvent strength measurements correlate well. The dielectric constant of methanol-carbon dioxide mixtures (50 °C and 110 bar) decrease linearly with added carbon dioxide until 0.45 mole fraction methanol is added. Further addition of carbon dioxide to methanol decreases the dielectric constant, but at a slower rate. The dielectric constant with 0.60 mole fraction methanol was nearly half that of pure methanol (10).
Figure 1 shows the variation of solvent strength when carbon dioxide is added to a 0.70:0.30 mole ratio methanol-water mixture at 25 °C and 172 bar (11). Similarly, carbon dioxide can be added to other solvent systems with minimal loss in solvent strength. However, the unique attribute of these data is that the hydrogen bond basicity of the ternary mixtures appears to increase with added carbon dioxide.
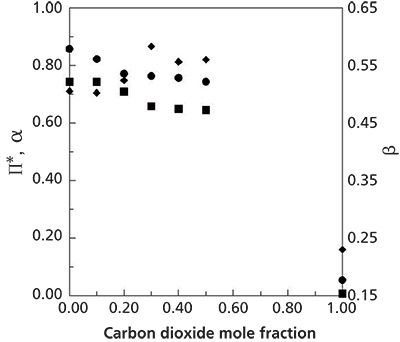
Figure 1: Variation of Kamlet-Taft solvatochromic parameters for methanol–water–carbon dioxide mixtures as a function of added carbon dioxide with the mole ratio of methanol–water held at 2.3 at 25 °C and 172 bar (• = A, ♦ =E, â = π*). Data adapted from reference 11.
Buffers
When high proportions of carbon dioxide are added to liquids, the solvents become weakly acidic unless other additives are included to control the pH. Buffers can be readily produced in these mixtures. We previously illustrated that buffers could be produced in methanol–water–carbon dioxide mixtures with pH values from 2.2 to 6.8 (12). The addition of carbon dioxide to methanol–water mixtures with a mole ratio of 69:31 produces a buffer with pH varying from 4.54 to 4.73 depending on the proportion of carbon dioxide added. The formation of carbonic acid and the presence of dissolved carbon dioxide provides the buffering components of this system. This buffer is very interesting because nonvolatile buffer additives are not necessary. The other interesting aspect of this study was that for the methanol–water–carbon dioxide mixtures studied, increasing the pressure from 120 to 207 bar did not significantly impact the measured pH.
Diffusion Coefficients
Chromatographic band dispersion in liquid chromatography is typically highly influenced by the resistance to mass transfer between the mobile phase and the stationary phase, which is inversely proportional to the diffusion coefficient of the mobile phase. Therefore, for low band dispersion, high diffusion coefficients are desired. Diffusion coefficients of solutes in a number of enhanced fluidity liquids were measured. For the methanol–carbon dioxide mixtures at 25 °C and 172 bar, the diffusion coefficient of benzene increases by approximately 75% by adding carbon dioxide up to 50 mol% (1). With increasing carbon dioxide above 50 mol% the diffusion coefficients increase at a faster rate up to that of pure carbon dioxide.
The variation of solute diffusion coefficients in methanol–water–carbon dioxide mixtures are also nonideal (13). Temperatures in excess of 60 °C were needed to increase the diffusion coefficient of benzene to the same value as the addition of 0.30 mole fraction carbon dioxide (Figure 2). However, by increasing the temperature and adding carbon dioxide to this methanol–water mixture, the greatest benefit was observed. For a 0.70:0.30 mole ratio methanol–water mixture, the addition of 0.30 mole fraction carbon dioxide and an increase in temperature to 58 °C caused a ninefold increase in the diffusion coefficient of benzene.
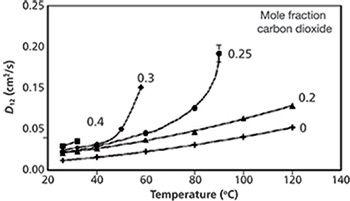
Figure 2: Variation of the diffusion coefficient of benzene at 138 bar in 0.70:0.30 methanol–water (+), 0.56:0.24:0.20 (â²), 0.52:0.23:0.25 (•), 0.49:0.21:0.30 (♦), 0.42:0.18:0.40 (â ) methanol–water–carbon dioxide. Data adapted from
Viscosity
Foster's group studied the change in viscosity in both methanol–carbon dioxide and ethanol–carbon dioxide mixtures. For methanol–carbon dioxide mixtures for pressures ranging from 12 bar to 78 bar and temperatures varying from 25 °C to 40 °C, the viscosity of the EFL decreased linearly to approximately 50% of the original viscosity from 0 to 50 mol% carbon dioxide (14). The viscosity continued to decrease with further addition of carbon dioxide, but not at the same rate. This change occurred at 50 mol% carbon dioxide for the entire temperature range. Ethanol–carbon dioxide mixtures were different; the viscosity decreased substantially with added carbon dioxide (15). However, the composition where the rate of change slowed varied with temperature. At 25 °C the reduction was linear up to 0.70 mole fraction carbon dioxide. For 30 °C, 35 °C, and 40 °C the rate of viscosity reduction slowed at 0.60, 0.45, and 0.25 mole fraction carbon dioxide, respectively. These data clearly show that the addition of carbon dioxide to conventional solvents will substantially impact the separation time.
Instrumentation
The instrumentation necessary to accomplish enhanced-fluidity chromatography is the same as that used in SFC with the condition that the software must allow use of more than 50% modifier. Alternatively, a conventional HPLC system can be used to deliver the organic solvents and a carbon dioxide compatible pump and mixer can be added at the outlet of the HPLC pump to allow for the use of carbon dioxide in the mobile phase. With this setup, a restrictor (small diameter tubing) must be added at the exit of the detector to control the pressure and flow rate of the mobile phase. For example, Sandra (16) used an Agilent 1200 HPLC system combined with a separate carbon dioxide pump and mixer at the outlet of the Agilent 1200 HPLC pumps. A stainless steel flow restrictor (4 m × 0.12 mm i.d.) and a needle valve were placed at the detector outlet to control the flow.
Commercial SFC instrumentation can be used under subcritical conditions all the way to EFLC conditions as long as the pressure limit of the instrument or the software for the solvent programming doesn't limit the mobile-phase conditions. (Note: These instruments use back-pressure regulators to control flow instead of fixed restrictors, as an example, an Agilent 1260 system can be used without modification for EFLC separations.)
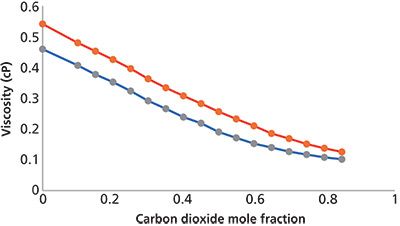
Figure 3: Variation of viscosity of a methanol–carbon dioxide mixture as a function of added carbon dioxide at 25 °C (red curve, pressure increased from 0 bar to 56.7 bar with increasing carbon dioxide) and 40 °C (blue curve, pressure increased from 1 bar to 76.7 bar with increasing carbon dioxide). Data adapted from reference 14.
Previous EFLC and a Range of Chromatographic Mechanisms
EFLC has been used effectively for reversed-phase, normal-phase, chiral, and size-exclusion modes of chromatography (17). In reversed-phase chromatography previous work has shown the separation time can be reduced by nearly half by adding carbon dioxide. Using normal-phase conditions for gradient polymer elution chromatography, Kawai also showed that the use of carbon dioxide in the mobile phase provided the highest resolution for poly(styrene-co-methyl acrylate) mixtures compared to the use of conventional liquids (30). In addition, chiral separations are typically faster and more efficient than in HPLC or SFC (17). As summarized by Guiochon and Tarafder (18), higher mobile-phase velocities, longer columns, and finer particles can be used with EFLC compared to HPLC with conventional solvents. These attributes are shared with high-temperature HPLC and ultrahigh-pressure liquid chromatography (UHPLC). The higher diffusion coefficients cause higher optimum mobile-phase velocities compared to HPLC and lower resistance to mass transfer, which increases the overall efficiency.
Separation of Polar Compounds - Subcritical Conditions Including EFLC Conditions
There continues to be considerable interest in providing fast and efficient separations for highly polar compounds. Taylor and coworkers (19) have been studying the use of subcritical chromatography for the separation of polar compounds with encouraging results. For example, Zheng and colleagues (19) described the separation of polypeptides up to 40-mers using a 2-ethylpyridine bonded silica stationary phase and carbon dioxide–methanol mobile phases with trifluoroacetic acid as an additive in the methanol to suppress the deprotonation of carboxylic acid groups and protonate the peptide amino acid groups on the protein. A 5–50% methanol gradient was used. Electrospray mass spectra with high signal-to-noise ratios were obtained with this technique and a lower separation time was achieved as well when comparing the optimized protein separations under SFC and HPLC conditions. Ashraf-Khorassani and Taylor (20) also showed that alcohol–carbon dioxide mixtures with up to 5% water using gradient conditions up to 50% alcohol were effective in separating the nucleobases, thymine, uracil, adenine, and cytosine.
Guillarme and coworkers (21) illustrated the value of subcritical fluid chromatography using a methanol–carbon dioxide mixture with 2–40% methanol at 40 °C and 150 bar with a 2-ethylpyridine column (100 mm × 3.0 mm, 1.7-μm dp Acquity UPC2 BEH-2-EP, Waters) or an Acquity BEH Shield RP18 hybrid column (50 mm× 2.1 mm, 1.7-μm dp, Waters) using 20 mM ammonium hydroxide in the mobile phase at a flow rate of 1.5 mL/min for the separation of highly polar compounds such as nucleobases and carbohydrates. A comparison of column types showed that gradient subcritical fluid chromatography could separate well at least 70% of the compounds studied with enhanced mass spectral sensitivity.
For chiral separations, Armstrong (22) evaluated subcritical conditions for chiral separations using macrocyclic glycopeptides. For the separation of polar analytes such as native amino acids using macrocyclic glycopeptides stationary phases, a combination of both acidic and basic modifiers and subcritical conditions with 48% to nearly 70% methanol were quite effective in providing excellent separations.
Sandra's group (16) was the first to study the possibility of using EFLC conditions for hydrophilic-interaction chromatography (HILIC). In this study, using a silica column (250 mm× 4.6 mm, 5-μm dp Zorbax Rx-SIL, Agilent Technologies) the five nucleobases, thymine, uracil, cytosine, guanine, and adenine were separated using 95:5 (v/v) ethanol–20 mM ammonium formate buffer at pH 3.0 combined with carbon dioxide. Increasing amounts of carbon dioxide increased the elution window and the separations were similar to those obtaining using acetonitrile–water mobile phases. Plate counts of 16,200 and 19,000 were obtained for 3 mL/min and 0.9 mL/min conditions.
Our group studied EFLC-HILIC separation of RNA nucleosides (adenosine [A], uridine [U], cytidine [C], and guanosine [G]) using EFLC mobile phases under isocratic conditions (23). Using a 150 mm × 4.6 mm, 3-μm dp Tosoh amide column and a 90:10 methanol–20 mM acetate buffer mobile phase, the addition of increasing proportions of carbon dioxide caused increased retention with a slight increase in band dispersion for the chromatographic bands. For example, the addition of 0.2 mole fraction carbon dioxide causes changes in the retention factor for A, C, U, and G of 72%, 66%, 97%, and 138%, respectively. With 20 mol% carbon dioxide, a separation time of 15 min was possible with resolution values >4 for all pairs. Alternatively, under optimized HPLC conditions with the same column using a 90:10 acetonitrile–20 mM acetate buffer, a complete separation was achieved in approximately 50 min.
To attempt separations of even more polar compounds, the separation of mono-, di-, and triphosphorylated nucleosides were studied (24). These compounds are typically separated using affinity chromatography, ion-exchange, or electrophoresis (25–27). A 150 mm × 4.6 mm, 3.5-μm dp Waters X-bridge amide column was used with 90:10 methanol–aqueous mobile phases buffered with ammonium phosphate. Often, phosphorylated compounds have poor peak shapes because of strong tailing in a broad range of chromatographic retention mechanisms using silica as a support. Phosphorylated compounds are strong hydrogen bond bases that interact with free silanols on a silica column through very strong hydrogen bonding interactions (28). The slow kinetics of desorption are the likely cause of the peak asymmetry. Strong bases such as triethylamine (TEA) are often added to the mobile phase to compete with the interactions with the free silanols (29). Two bases that are widely used in organic synthesis and described as superbases because of their strong basicity in both aqueous and organic solvents were also considered and compared to TEA: 1,4-diazabicyclo [2.2.2] octane (DABCO) and 1,5- diazabicyclo [4.3.0] non-5-ene (DBN).
Figure 4 compares the efficiency and the peak asymmetry for the mono- and diphosphorylated nucleosides obtained with TEA, DABCO, and DBN in a mobile phase that contained ammonium dihydrogen phosphate buffer under LC conditions (Figure 4a) and with the addition of 0.1 mole fraction carbon dioxide to the mobile phase with a mobile-phase velocity of 1 mL/min. When compared to the data where no base was added, DBN provides the best results with a 12% to 18% increase in efficiency in LC for monophosphate nucleotides and 111% to 320% for the diphosphates. In EFLC, the efficiency increased from 9% to 39% for monophosphates, and from 67% to 115% for diphosphates. For both HPLC and EFLC, tailing was the major contributor to low efficiency for the phosphorylated compounds; the impact of base addition on the asymmetry of the peak was studied to better understand the impact of each base on efficiency. DBN provided both the greatest decrease in peak asymmetry and increase in efficiency, followed by DABCO. Both bases greatly improved the peak shape while marginally affecting the retention of the analytes; no significant decrease in k was observed in EFLC, and an average 10% decrease in k was observed in LC. TEA either increased the peak asymmetry or did not impact it significantly.
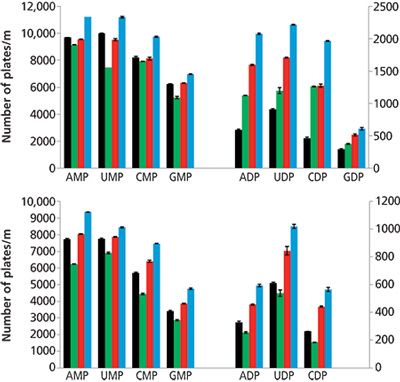
Figure 4: Effect of different bases on the efficiency of the nucleotides: (a) in LC and (b) with 0.1 mole fraction carbon dioxide added. Conditions: 5 mM of each base was added to a 75 mM ammonium phosphate solution, which was used to prepare the 90:10 (v/v) methanol–aqueous mobile phase. The y-axis on the left corresponds to the efficiency values for the monophosphate compounds, the axis on the right corresponds to the values for the diphosphate compounds. No base (black bars), TEA (green bars), DABCO (red bars), and DBN (blue bars). Data adapted from reference 24.
Similar to other HILIC separations of polar compounds, the addition of a salt to the mobile phase was quite important. Sodium chloride (0.02 M) was used to assist the formation of the adsorbed water phase, and 75 mM ammonium phosphate was used to decrease the retention of the phosphate groups. Figure 5 shows that carbon dioxide expands the elution window for the compounds.
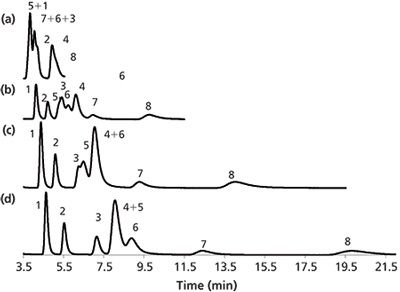
Figure 5: Separation of various nucleosides and nucleotides: (a) LC, (b) 0.15 mole fraction of carbon dioxide, (c) 0.20 mole fraction of carbon dioxide, and (d) 0.25 mole fraction of carbon dioxide. Mobile phase: 90:10 methanol–75 mM ammonium phosphate + 5 mM DBN. Peaks: 1 = adenosine, 2 = cytidine, 3 = uridine, 4 = guanosine, 5 = AMP, 6 = CMP, 7 = UMP, 8 = GMP. Data adapted from reference 24.
Summary
Now that SFC chromatographic instruments have a much larger range of operating pressures with accompanying precise temperature control, it is time to consider using liquefied gases such as carbon dioxide in mobile phases for a broader range of chromatographies. The lower viscosity of mobile phase and increased diffusivity for analyte separations using 0–100% liquefied gas provides decreased analysis time and often improved efficiency. The entire range of this solvent continuum is valuable for separation science. A continuous change in viscosity, solvent strength, diffusivity, and permittivity occurs across this range of mobile-phase compositions. In particular, EFLC mobile phases are also providing value for the separation of highly polar compounds with similar operating parameters as in conventional HPLC. While advantages in performance are often noted when using acetonitrile–water for reversed-phase HPLC and HILIC compared to alcohol–water mixtures, the addition of carbon dioxide to the alcohol–water mixtures improves the chromatographic performance significantly. Also, both alcohol–carbon dioxide and alcohol–water–carbon dioxide are environmentally friendly.
Susan V. Olesik is the Dow Professor and Chair in the Department of Chemistry and Biochemistry at Ohio State University in Columbus, Ohio. Direct correspondence to: olesik.1@osu.edu
References
(1) Y. Cui and S.V. Olesik, Anal. Chem. 63, 1813–1819 (1991).
(2) C.J. Chang and A.D. Randolph, AIChE J. 36, 939–942 (1990).
(3) H. Yuan and S.V. Olesik, Anal. Chem. 70, 1595–1603 (1998).
(4) J. Zhao and S.V. Olesik, J. Chromatogr. A 923, 107–117 (2001).
(5) H. Chen and Cs. Horváth, J. Chromatogr. A 705, 3–20 (1995).
(6) G. Guiochon, Optimization in Liquid Chromatography in High Performance Liquid Chromatography: Advances and Perspectives, Vol. 2, C. Horvath, Ed. (Academic Press, New York, 1980), pp. 1–56.
(7) F. Erni, J. Chromatogr. 282, 371–382 (1983).
(8) H. Yuan and S.V. Olesik, J. Chromatogr. Sci. 35, 409–416 (1997).
(9) T. Aida and H. Inomata, Mol. Simulat. 30, 407–412( 2004).
(10) S.B. Lee, R.L. Smith, H. Inomata, and K. Arai, Rev. Sci. Instr. 72, 4226–4230 (2000).
(11) Y. Cui and S.V. Olesik, J. Chromatogr. A 691, 151–162 (1995).
(12) D. Wen and S.V. Olesik, Anal. Chem. 72, 475–480 (2000).
(13) S.T. Lee and S.V. Olesik, Anal. Chem. 66, 4498–4506 (1994).
(14) R. Sih, F. Dehghani, and N.R. Foster, J. Supercrit. Fluids 41, 148–157 (2007).
(15) R. Sih, M. Armenti, R. Mammucari, F. Dehghani, and N.R. Foster, J. Supercrit. Fluids 43, 460–468 (2008).
(16) A. dos Santos Pereira, AJ. Girón, E. Admasu, and P. Sandra, J. Sep. Sci. 33, 834–837 (2010).
(17) S.V. Olesik, Adv. Chromatogr. 46, 424–449 (2008).
(18) G. Guiochon, and A. Tarafder, J. Chromatogr. A 1218, 1037–1114 (2011).
(19) J. Zheng, J.D. Pinkston, P.H. Zoutendam and L.T. Taylor, Anal. Chem. 78, 1535–1545 (2006).
(20) M. Ashraf-Khorassani and L. Taylor, J. Sep. Sci. 33, 1682–1690 (2010).
(21) A. Periat, A. Grand-Guillaume, and D. Guillarme, J. Sep. Sci. 36, 3141–3151 (2013).
(22) Y. Liu, A. Berthod, C.R. Mitchell, T.L. Xiao, B. Zhang, and D. Armstrong, J. Chromatogr. A 978, 185–204 (2002).
(23) J.W. Treadway, G.S. Philibert, and S.V. Olesik, J. Chromatogr. A 1218, 5897–5902 (2011).
(24) G. Philibert and S.V. Olesik, J. Chromatogr. A 1218, 8222–8230 (2011).
(25) M. Hossel, M.G. Corneliu, J. vom Brocke, and H.H. Schmeiser, Electrophoresis 31, 299–302 (2010).
(26) N.J. Alves, S.D. Stimple, M.W. Handlogten, and J.D. Ashley, Anal. Chem. 84, 7721–7728 (2012).
(27) N. Tomiya, E. Ailor, S.M. Lawrence, M.J. Betenbaugh, and Y.C. Lee, Anal. Biochem. 293, 129–137 (2001).
(28) E.D. Davis, W.O. Gordon, A.R. Wilmsmeyer, D. Troya, and J.R. Morris, J. Phys. Chem. Lett. 5, 1393–1399 (2014).
(29) D.V. McCalley, Adv. Chromatogr. 46, 305–350 (2008).
(30) E. Kawai, K. Shimoyama, K. Ogino, and H. Sato, J. Chromatogr. A 991, 197–203 (2003).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.











