Electrical Potential as a Driving Force in Sample Preparation
Entirely new sample preparation technologies continue to be introduced, mainly in the academic sector. Some of these technologies will undoubtedly stay in the academic laboratory. However, some new technologies may "cross the chasm" and eventually become a standard laboratory procedure. This instalment will examine some of those methods.
To achieve lower detection limits and better selectivity, researchers are always exploring new ways to enhance sample preparation and separation technology. The addition of electrical potential to existing technologies or for entirely new approaches is relatively unexplored but can create another dimension of selectivity in sample preparation. In addition, speed and sensitivity are added benefits. This instalment looks at the use of electroenhancement to solid-phase extraction, solid-phase microextraction, and membrane and liquid extraction.
We are all familiar with the role of electromigration in enhancing separations in capillary and gel electrophoresis and related techniques and in capillary electrochromatography. In addition, electrical potential has been used in electrochemical and other forms of chromatographic detection. The use of electrical potential to enhance and add another dimension of selectivity to sample preparation is still a relatively new, noncommercialized technique. The purpose of this instalment is to discuss how electrically driven enhancement has already proven to offer some major advantages to the preparation of samples whose analytes of interest are ionized or can become ionized by the appropriate adjustment of pH to the separation media. We explore five sample preparation methods where the application of a charge across barriers provides enhanced selectivity, often leaving undesired matrix and impurities behind, thereby accomplishing the goals of sample preparation.
The main purpose of sample preparation is to transport the analytes of interest into a medium that is compatible with the analysis method, to remove interferences, and to concentrate the analytes so that they can be more easily detected and quantitated. Sample preparation using electrolytic potential in bioanalysis is particularly attractive since many of the water-soluble compounds encountered are charged or could be made charged.
Electroextraction
Most of us are familiar with liquid–liquid extraction (LLE) because it is still one of the most popular techniques used for sample cleanup. In LLE, two immiscible liquids are used to partition analytes from a sample into one of the two phases, an organic phase and an aqueous phase. After shaking the phases together in a separatory funnel, analytes that prefer the organic medium are partitioned into it while those analytes that prefer the aqueous media, usually polar compounds or ionized compounds, are partitioned into it. Electroextraction (EE) is a sample enrichment technique that focuses charged analytes from a large volume of one phase into a small volume of aqueous phase through the application of an electric current. In two-phase EE, the immiscible phases that are electrically conductive are kept between electrodes, and upon addition of an electric field, charged particles travel from one phase to another, thereby separating anions and cations, as depicted in Figure 1. Three-phase systems are also used where anions and cations are attracted into the two outer phases leaving uncharged particles in the middle phase. Generally, organic solvents need to contain a small amount of water so that they can be made conductive. In two-phase EE, a rapid electromigration takes place because ions in the organic phase are subjected to very high electric field strength resulting from low conductivity. Because the electric field of the aqueous acceptor phase is much lower, ions in the organic phase will migrate at high velocity to be concentrated just beyond the liquid–liquid interface.

Figure 1: Diagram showing the principle of electroextraction.
The apparatus used to conduct electroextraction consists of a vial with a conical bottom, a bottom grounded electrode in contact with the lower aqueous layer and a capillary (injection port) to inject the sample solution, and an upper electrode in contact with the upper organic phase. The EE experiment can also be performed in an electrophoresis-like capillary and this version is referred to as capillary electroextraction (cEE). Lindenburg and colleagues (1) used a wide-bore capillary connected to a two-way, 10-port switching valve interfaced to a liquid chromatography–mass spectrometry (LC–MS) system. This system allowed up to 100 μL of sample to be extracted, and they showed improved detection of peptides in an unspiked plasma sample. For these real samples, limits of detection values were in the 10–50 nM range for several angiotensin peptides, which indicates the potential for cEE as an on-line sample concentrating technique. In another application, this same laboratory applied cEE–LC–MS to urine metabolites including amino acids and acylcarnitines (2).
Three-Phase Electroextraction
A recent variation of electroextraction termed three-phase electroextraction also uses the principles of EE (3). In this technique, charged analytes migrate from an aqueous donor phase through an immiscible organic filter phase into an aqueous acceptor phase by applying an electric field between the donor and acceptor. In principle, the experiment works like electromembrane extraction (EME) (see next section), but the intermediate organic phase isn't immobilized in a membrane. The extraction occurs when the electric field is applied between the aqueous donor and the aqueous acceptor phase (shown as the green drop suspended in the organic filter phase in Figure 2). In this experiment, no stirring is required, as is the case for single-drop microextraction, and a rapid, complete extraction is accomplished. A fairly large donor volume can be used and a small acceptor volume (microlitre to submicrolitre) is possible, thereby providing large enrichment factors and allowing direct injection into a nano electrospray ionization (ESI) MS source. By choosing an optimized organic filter phase, selectivity of the extraction can be improved. Enrichment factors were found to be related to the polarity of the organic filter phase with relative standard deviation (RSD) values less than 15%. Also, as the polarity of the analyte increases, the extraction rate increases, with the partition coefficient across the aqueous–organic filter interface being the limiting factor. Large molecules like proteins will not be transported into the acceptor phase through the organic filter phase. Thus, the three-phase electroextraction technique could be quite useful for bioanalysis.
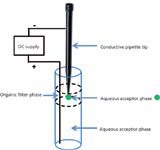
Figure 2: Setup for three-phase electroextraction.
In their recent paper, Raterink and coworkers (3) demonstrated that model compounds (carnitines) could be extracted from a 50-μL sample into a small 2-μL acceptor droplet in only 3 min. Samples of acylcarnitines were spiked into a human blood plasma sample and were found to be effectively extracted, but enrichment factors were slightly lower compared to model analytes, presumably because of protein binding. Calibration curves for the spiked carnitines in human plasma showed linearity over two orders of magnitude. A proof of principle for the on-line integration in an automated nanoESI-direct infusion-MS platform was demonstrated. These preliminary results showed that three-phase EE may prove to be an automatable, novel sample pretreatment for high-throughput bioanalysis.
Electromembrane Extraction
A number of years ago, S. Pedersen-Bjergaard and K.E. Rasmussen of the University of Oslo demonstrated that the use of a 300-V electrical potential imposed across a thin (200 μm) supported liquid membrane for 5 min assisted the electrokinetic migration of drug substances from a 300-μL donor compartment to 30 μL of an acceptor solution (4). The membrane was impregnated with 2-nitrophenyl octyl ether immobilized in the pores, making the experiment a form of liquid–liquid–liquid membrane extraction with an added electrical potential. The setup was used to isolate and concentrate a series of basic drugs in an acidified solution of water, human plasma, and human urine. Enrichment factors were in the range of 7.0–7.9. At the time, they speculated that this approach could become "an interesting tool for future isolation within chemical analysis". Since then, they and other research groups have applied this priniciple to not only flat supported liquid membranes (SLMs), but also to inexpensive, supported hollow-fibre membranes that make it experimentally more convenient for small volumes.
In 2010, Astrid Gjelstad from the above-mentioned University of Oslo group published a paper in LCGC (5) demonstrating the use of such a system for the enrichment of pethidine, a basic drug, in aqueous solution. The EME setup that she used is illustrated in Figure 3. The setup and procedure is very similar to hollow-fibre liquid-phase microextraction (HF-LPME) (6,7), but in EME electrodes are inserted into the sample and the acceptor solution, respectively, and the electrodes are connected to a direct current electrical power supply. In EME, the driving force for the extraction is the electrical potential, and this parameter must be optimized. Normally, extraction recoveries increase with increasing voltage up to a certain level, after which there is no further gain in recovery versus voltage (5). The optimal voltage must be established by experimental optimization, as this voltage is dependent on both the analytes and the composition of the SLM. In EME, an electrical potential is applied over the electrodes of typically 5–100 V, creating an electrical field over the SLM. For EME of basic analytes, the anode is located in the donor sample solution, whereas the cathode is placed in the acceptor solution. Both the donor and acceptor solutions should be acidic (dilute hydrochloric or formic acid). The sample must be acidified to make sure that the basic analytes are ionized. Thus, the basic analytes are extracted as protonated species from the sample, through the SLM, and into the acceptor solution. The acceptor solution is also acidic to support the electro-kinetic transfer and to avoid back-extraction into the SLM. For acidic analytes, the direction of the electrical field is reversed, and alkaline conditions (dilute sodium hydroxide or ammonia solution) are used in the sample and the acceptor solutions to maintain the analytes in their charged configuration. EME is more rapid than HF-LPME because the driving force is an electrical potential rather than a pH gradient. The EME experiment can often be completed in under 5 min.
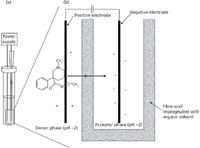
Figure 3: Diagram of (a) the EME setup and (b) principle of operation using pethidine as a model compound (5).
For EME of basic substances, immobilized solvents like 2-nitrophenyl octyl ether, 1-ethyl-2-nitrobenzene, and 1-isopropyl-4-nitrobenzene are typically used for the SLM (5). For extraction of more polar substances, these solvents are normally added to an ion-pair reagent or another modifier to facilitate the mass transfer of analyte across the SLM. Typical examples are di-(2-ethylhexyl) phosphate and tris-(2-ethylhexyl) phosphate (5). Acidic compounds have only been extracted a few times by EME, and in these cases 1-octanol has been used as supported liquid. Several reviews have been published summarizing applications of EME (8–12).
Electroenhanced Solid-Phase Microextraction
Solid-phase microextraction (SPME) is a popular extraction technique used in gas, liquid, and headspace sampling and analysis. Research groups have been investigating ways to make the technique even more selective and sensitive. A recent innovation has been the application of an electric potential to commercial SPME fibres (13). The technique has been called electroenhanced solid-phase microextraction (EE-SPME). In this approach, the fibres were used with the direct immersion mode in aqueous samples using an applied potential to extract methamphetamine. The experimental setup is depicted in Figure 4. When used with an applied voltage of 12 V, the fibre coated with carboxen-polydimethylsiloxane (CAR-PDMS) was more efficient for methamphetamine than those constructed of polyacrylate, PDMS, or PDMS-divinylbenzene. The CAR-PDMS gave sixfold greater sensitivity than the polyacrylate and 1.5-fold greater sensitivity than the other two fibres. After this optimum fibre was established, the workers went on to optimize the solution pH (pH 7), applied voltage (12 V), extraction time (20 min), and stirring speed. Overall, the EE-SPME technique gave a more efficient extraction for methamphetamine compared to the use of regular SPME — a 159-fold higher enrichment factor. The final analysis was performed using thermal desorption and gas chromatography–mass spectrometry (GC–MS). The calibration plot under the best selected parameters was linear in the range of 0.5–15 ng/mL (r = 0.9948). For the analysis of methamphetamine in human urine samples, the limit of detection (LOD) was 0.25 ng/mL with a satisfactory RSD of 6.12% (n = 3). Recovery of methamphetamine in the spiked urine samples was 86.9%.
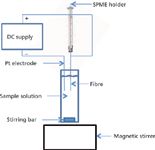
Figure 4: Experimental setup for electroextractionâSPME.
Electric Field-Assisted Solid-Phase Extraction
Solid-phase extraction (SPE) is one of the most popular sample preparation techniques used before chromatographic analysis. It relies on similar principles of retention-elution that are characteristic of liquid chromatography so a number of mechanisms (that is, reversed phase, normal phase, anion and cation exchange, affinity, and so on) are available to the chemist. The application of electric potential as an additional force for the SPE of ionizable or ionic compounds was investigated by Brazilian workers (14). The modified syringe-type SPE cartridge used in their studies is shown in Figure 5. In the final assembly, the various elements were closely sandwiched together. They termed the technique E-SPE. In their investigations of E-SPE, they studied the sorption or elution behaviour of a model compound, the antimicrobial marbofloxacin, using both conventional C18 SPE and electric field-assisted C18 SPE. The electrical potential was applied only during the wash step, but this step was considered the most important step to obtain adequate cleanup and extraction efficiencies.
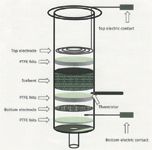
Figure 5: Modified SPE cartridge for studies of electric field-assisted SPE. Shown is an expanded view; the final assembly is tightly sandwiched to eliminate dead volume. (Courtesy of Professor Susanne Roth, University of Campinas, Brazil.)
The application of electric potential across an SPE cartridge wasn't as straightforward as they expected. Similar to the development of any new technique, they uncovered issues that had to be addressed in the final design. Bubble formation at the electrodes and pH changes because of electrolysis of water, temperature increases, and electro-osmotic flow affected by silanol content of the SPE sorbent were a few of the problems they encountered. They were able to work around some of these problems by optimizing the design of the electrodes to minimize bubble formation. In a buffered system, there were no observed pH changes during the E-SPE experiment. For unbuffered wash systems, they came up with a clever bidirectional flow modification (not shown here), that negated the pH changes that resulted from hydronium formation during electrolysis by introducing solvent into the cartridge at a higher flow rate than the solution leaving the cartridge (15). This solvent neutralized and diluted the ions generated during electrolysis. Temperature increases inside the cartridge were no more than 8 °C. They were able to reverse the polarity in real time and minimize the effects of electro-osmotic flow. Using a flow extraction system that they developed, they were able to control the flow rate, electric current, electrical potential, and temperature inside of the cartridge.
The authors compared the recovery with and without the application of electrical potential across the packed bed. For the model compound marbofloxacin, recovery was improved by 2.3 times or reduced 4.2 times in comparison to conventional SPE when the top electrode was used as the cathode or anode (by reversing polarity), respectively. The results demonstrated that the electric field acted as an additional driving force and contributed to enhance extraction efficiency and selectivity in the SPE procedures.
Conclusion
Although commercially the area of electroenhancements to sample preparation protocols is relatively undeveloped, the use of electric potential can create additional selectivity, sensitivity, and speed as was shown here using a few published examples. The electrical enhancement techniques are especially applicable to situations where the analytes of interest are ionic or ionizable in dilute solution, such as might be encountered for drugs and their metabolites in biological fluids. The technique of three-phase electroextraction looks particularly promising for this application, especially when the feasibility of automation has been demonstrated. There are other areas where electrical potential can aid sample preparation, such as the focusing of analytes before capillary electrophoresis and electrofiltration. These techniques may be discussed in future "Sample Preparation Perspectives" instalments.
"Sample Preparation Perspectives" Editor Ronald E. Majors is an analytical consultant and is a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should be addressed to "Sample Preparation Perspectives", LCGC Europe, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com
References
(1) P.W. Lindenburg, F.W.A. Tempels, U.R. Tjaden, J. van der Greef, and T. Hankemeier, J. Chromatogr. A 1249, 17–24 (2012).
(2) P.W. Lindenburg, U.R. Tjaden, J. van der Greef, and T. Hankemeier, Electrophoresis 33, 19–20 (2012).
(3) R.-J. Raterink, P.W. Lindenburg, R.J. Vreeken, and T. Hankmeier, Anal. Chem. 85, 7762–7768 (2013).
(4) S. Pedersen-Bjergaard and K.E. Rasmussen, J. Chromatogr. A 1109, 183–190 (2006).
(5) A. Gjelstad, LCGC North Am.28(2), 92–112 (2010).
(6) S. Pedersen-Bjergaard and K.E. Rasmussen, Anal. Chem. 71, 2650–2656 (1999).
(7) S. Pedersen-Bjergaard and K.E. Rasmussen, J. Chromatogr. A 1184, 132–142 (2008).
(8) M. Balchen, L. Reubsaet, and S. Pedersen-Bjergaard, J. Chromatogr. A 1194, 143–149 (2008).
(9) M. Balchen, T.G. Halvorsen, L. Reubsaet, and S. Pedersen-Bjergaard, J. Chromatogr. A 1213, 14–17 (2009).
(10) C. Basheer, S.H. Tan, and H.K. Lee, J. Chromatogr. A 1213, 14–18 (2008).
(11) J. Lee, F. Khalilian, H. Bagheri, and H.K. Lee, J. Chromatogr. A 1216, 7687–7693 (2009).
(12) L. Xu, P.C. Hauser, and H.K. Lee, J. Chromatogr. A 1214, 17–22 (2008).
(13) T. Yin Tan, C. Basheer, M.J. Yan Ang, and H.K. Lee, J. Chromatogr. A 1297, 12–16 (2013).
(14) R. M. Orlando, J. J. Rodrigues-Rohwedder and S. Rath, Chromatographia On-Line Publication DOI 10.1007/s10337-013-2565-9, 28 September 2013.
(15) http://biq.iqm.unicamp.br/arquivos/teses/ficha94196.htm.

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










