The Development of the Amino Acid Analyzer
LCGC North America
This installment of "Milestones in Chromatography" discusses the events leading to the development of the amino acid analyzer near the end of the 1950s at the Rockefeller Institute of Medical Research by Moore, Stein, and Spackman.
In previous columns, we have discussed the development of the first gel-permeation chromatograph (1) and the work by Csaba Horváth at Yale University (New Haven, Connecticut) in the laboratory of Professor S.R. Lipsky, leading to the first modern, high-pressure liquid chromatograph (2). However, although these instruments usually are called the first liquid chromatography (LC) instruments, they actually were preceded by another complex instrument that was based upon the principles of LC on ion-exchange resins: the automated amino acid analyzer developed in 1958 at The Rockefeller Institute for Medical Research (New York) by S. Moore, W.H. Stein, and D.H. Spackman (3,4).
The amino acid analyzer opened an entirely new field for research, permitting the elucidation of the composition of proteins. We might even say that the rapid expansion of biochemistry would have been impossible without it. However, because the majority of chromatographers are not in the biochemical field, this development is relatively unknown to the general public. In this installment, we try to fill this gap in the history of chromatography.
To understand the importance of this development, a brief summary of proteins and amino acids, and the development of separation based upon ion-exchange, is useful.
Amino Acids and Proteins
Amino acids are the building blocks of proteins: very complex, mostly α-helical, three-dimensional polymers consisting of hundreds of amino acids connected by peptide bonds. Proteins are the key gene-expressed compounds in life processes, and this is reflected by their name: "protein" comes from the Greek word proteios, meaning "of primary importance." This term was first used in 1839 by the great Swedish chemist JÖrs Jakob Berzelius (1770–1848). The study of the amino acids has a long history, leading to the slow recognition of the individual amino acids. The 1902 Nobel laureate Emil Fischer (1852–1919) showed in the early 1900s how amino acids are bound to each other forming polypeptides, the building blocks of proteins (5).
In the first part of the twentieth century, research on proteins and amino acids was carried out along two lines: as part of nutrition studies and investigating their chemistry and composition. Decades of painstaking work by many researchers established that the proteins of the humans and animals contain 20 amino acids, from which 10 cannot be synthesized by the human body, but must be taken nutritionally. The proper amount and balance of the essential amino acids in our food intake is very important. However, these 20 natural amino acids are only part of the amino acids occurring in nature: we know of the existence of hundreds, or even thousands of additional ones.
Naturally, researchers in biochemistry, nutrition, and other life sciences fields wanted to identify the individual amino acids present in proteins and determine their quantitative levels. For this, the proteins must first be hydrolyzed to break the peptide bonds. This usually is done by boiling the peptide with an acid for an extended time; it is a delicate operation, as some amino acids can be destroyed during hydrolysis, and various safeguards are used to prevent and compensate for it.
Various methods have been developed for the determination of the individual amino acids. Emil Fischer was the first to show that the esters of the amino acids can be distilled, and he used the fractional distillation of the esters prepared from protein hydrolysates to obtain a more precise understanding of their original composition. Other methods introduced in the first part of the twentieth century involved selective precipitation as insoluble salts, colorimetric analysis, and microbiological assays, but these were complicated and time-consuming operations (6). A.J.P. Martin and R.L.M. Synge first described partition chromatography in 1941 for column chromatography (7) and then its paper chromatography version — introduced in 1944 by Martin's group (8) — represented a major breakthrough, permitting separation and simultaneous determination of amino acids in a straightforward manner. Martin and Synge received the 1952 Chemistry Nobel Prize for the invention of partition chromatography.
Using partition chromatography on columns packed with starch, in 1944 Synge started to establish the sequence of amino acids in peptides, leading to the structure elucidation of gramicidin S, a cyclic decapeptide (9–12). In the subsequent 10 years, Frederick Sanger at Cambridge University (Cambridge, United Kingdom) further advanced Synge's methodology, finally succeeding in the establishment of the sequence of the 51 amino acids forming the molecule of insulin (13). His achievements were recognized with the 1958 Nobel Prize in Chemistry.
Ion-Exchange Chromatography
Ion exchange in soils was first described in 1850, but for decades, only sodium aluminosilicates ("zeolites") were available as exchange material. Thus, the possible utilization of the phenomenon was severely limited. In the late 1930s at Columbia University (New York), Harold Urey tried to separate lithium and potassium isotopes on very long (30–100 ft) columns filled with zeolite and was able to achieve partial separation (14). A new area in ion-exchange began in 1935 when B.A. Adams and E.L. Holmes (National Chemical Laboratories, Teddington, United Kingdom) prepared synthetic organic ion-exchange resins (15). These were first used in Sweden by Olof Samuelson in 1939 (16,17). Such synthetic polymeric ion-exchange resins also were produced in the United States by Rohm & Haas (Bridesburg, Pennsylvania) and Dow Chemical Co. (Midland, Michigan) under the respective trade names of Amberlite IR-100 and Dowex 50.
Such synthetic resins were used extensively during World War II — even on a preparative scale — as part of the Manhattan Project (the development of the atomic bomb) for the separation of fission products and rare earths. This work was carried out at Iowa State University (Ames, Iowa) and Clinton National Laboratories (Oak Ridge, Tennessee) and was highly classified: its publication was forbidden for years. Finally, at the Fall 1947 National Meeting of the American Chemical Society (September 17, 1947), a special symposium was held where members of the two teams presented detailed reports on their work. Parallel to the symposium, Chemical & Engineering News published a report on the activities carried out at Ames and Oak Ridge (18). The text of the 13 papers that were presented at the symposium was published in November 1947 as a special issue of the Journal of the American Chemical Society. In 1949, at the Chromatography Conference of the Faraday Society in London, the two team leaders, F.H. Spedding of Ames and E.R. Tompkins of Oak Ridge, presented detailed reports on the whole project. This was how the scientific community learned about the possibilities of the use of modern ion exchangers. (A detailed summary of the work carried out as part of the Manhattan Project was published in 1999 in an installment of LCGC's "Milestones in Chromatography" column [19].)
Amino Acid Research at the Rockefeller Institute
The research leading to the development of the amino acid analyzer was carried out at The Rockefeller Institute for Medical Research. This institute was founded in 1901 by John D. Rockefeller (1839–1937) and built on farmland along the East River. The first laboratory opened in 1904 and its hospital for the study of human diseases was established in 1910. Soon after, the institute developed into one of the principal research organizations in biomedical sciences. The institute started to grant Ph.D. degrees in 1954, taking the status of a graduate university, and finally, in 1965, it changed its name to Rockefeller University. Because our story is related to the period before 1960, we shall use the name of Rockefeller Institute in our narrative.
In March 1933, Hitler and his Nazi party came to power in Germany. A number of Jewish scientists soon had to leave the country, many of them immigrating to the United States. A prominent scientist involved in this intellectual migration was Max Bergmann.
Max Bergmann (1884–1944) was a student and associate of Emil Fischer and had been involved in his amino acid research. He had a distinguished career in Germany, was a founder and director of the Kaiser Wilhelm Institute for Leather Research (Dresden), which he developed into a world-renowned, leading center for protein chemistry research in the 1920s. He left Germany in 1933, joining the Rockefeller Institute, where he soon established a laboratory and became a central figure in protein research in the United States. Bergmann surrounded himself with the most talented young, postdoctoral scientists, launching them to become important members of the international group of protein chemists. Two who are the key figures in our story are William H. Stein and Stanford Moore (Figure 1).

Figure 1
William H. Stein (1911–1980) studied at Columbia University, graduating in 1937 with a thesis on the analysis of the amino acids of the protein elastin. He then went directly to Bergmann as a postdoctoral associate.
Stanford Moore (1913–1982) studied at Vanderbilt University (Nashville, Tennessee) and the University of Wisconsin (Madison), graduating with a thesis on the characterization of carbohydrates as benzimidazole derivatives. It is interesting to note that during his undergraduate years, Moore also took engineering courses (in fact, his first thought was to major in engineering) and as a graduate student in the laboratory of Professor Karl P. Link, at the University of Wisconsin (Madison, Wisconsin), he received an extensive training in microchemistry, including Pregl's microanalytical methods. After graduating in 1939, he joined Bergmann at the recommendation of Professor Link.
There were two main lines of investigation in Bergmann's laboratory: the field of proteolytic enzymes and the structural chemistry of proteins. Both Moore and Stein had been involved in the second field and their task was to further improve gravimetric methods of amino acid fractionation through the formation of sparingly soluble salts (20–22). America's entry into the war interrupted their work: Bergmann's group started to work for the Office of Scientific Research & Development (OSRD), investigating the physiological effects of mustard gas and related compounds, while Moore left the laboratory and served with OSRD in Washington and at the headquarters of the U.S. Armed Forces in the Pacific Area. At the end of the war, Moore returned to the Rockefeller Institute. Bergmann died suddenly in 1944 and his former group was dissolved, but the director of the institute allocated part of Bergmann's laboratories to Moore and Stein to develop their own research program. This is how their close cooperation began, and it lasted for 40 years, eventually leading to the quantitative analysis of amino acids and its automation, and culminating in the elucidation of the 124 amino acid sequence of ribonuclease, honored by the 1972 Chemistry Nobel Prize. As noted by Moore, "We approached problems with somewhat different perspectives and then focused our thoughts on the common aim. If I did not think of something, he was likely to, and vice versa, and this process of frequent interchange of ideas accelerated our progress in research" (23).
When Moore and Stein returned to peacetime research, the wartime issues of the British Biochemical Journal had just became available in the United States and there, they read about the work of Synge describing the separation of free amino acids by partition chromatography on columns containing starch as the stationary phase (10,11). They immediately adapted the technique to their investigations, further improving it. They collected column effluent in small fractions and by establishing the amount of amino acids in each fraction, constructed chromatograms showing separated peaks. Quantitation of each fraction was carried out by adapting the color reaction of the amino acids with ninhydrin. In the beginning, the small fractions were collected manually, but this was much too tedious due to the large number of fractions. Therefore, they developed a very sophisticated, fully automated fraction collector.
In November 1946, The New York Academy of Sciences held a two-day conference on chromatography and there, Moore and Stein presented a report on the early results of their work. The publication of the proceedings of the meeting was delayed by more than one year and, thus, in the printed text of their presentation they also could include a detailed description of their newly developed fraction collector (24). This was followed by four publications reporting on their further results and the determination of the amino acid composition of β-lactoglobulin and bovine serum albumin, using partition chromatography on starch columns (25–28).
The starch columns worked well, but they were very slow. Therefore, Moore and Stein were looking for other possibilities. Meanwhile, the detailed publications on ion-exchange chromatography became known (described previously) that led to the pioneering work of Waldo E. Cohn at Oak Ridge (Tennessee), on the separation of nucleic acid constituents by ion-exchange chromatography (29,30). Reports from England also involved ion-exchange chromatography for the separation of amino acids in protein hydrolysates, although in the displacement and not in the elution mode (31). These reports initiated intensive activities by Moore and Stein, who were joined by C.H.W. Hirs (born in 1923), who graduated from Columbia University in 1949. Dr. Hirs remained at the Rockefeller Institute for 10 years and participated in much of the amino acid research. He was the first young postdoctoral associate in Moore and Stein's group. In the next 20 years, 20 additional young scientists spent a few years with them, cooperating in their research and learning the intricacies of protein investigations.
Dr. Hirs provided a very vivid narrative of the intensive research carried out by Moore and Stein's group (32). Their first report on the use of ion-exchange resins for amino acid separation was published in 1951 (33), followed by six more papers in the early 1950s. Studying these, one can follow the careful, painstaking, and systematic investigations approaching every aspect of ion-exchange chromatography and the possibility not only of quantitative amino acid analysis, but also of separation on semipreparative scale.
The 1972 Chemistry Nobel Prize was awarded to Moore and Stein (together with C.B. Anfinsen) for their "contribution to the understanding of the connection between chemical structure and catalytic activity of the active centre of the enzyme ribonuclease." These investigations started in 1954 and aimed at the determination of the complete amino acid sequence of the molecule. In the course of these studies, a very large number of protein hydrolysate analyses had to be performed and the manual procedure, one complete analysis taking a few days, was just not satisfactory: analysis time had to be reduced and some kind of automation was needed. Moore always had been interested in engineering work — let us not forget that in college, he also took engineering courses. Thus, they decided to investigate the possibility of developing an automated amino acid analyzer. In this work, they had a new associate: D.H. Spackman (born 1924), who received the Ph.D. degree from the University of Utah (Provo, Utah) in 1954, and moved from there to the Rockefeller Institute.
The development proceeded along two lines: further improvement of the ion-exchange separation process and the construction of an instrument. With respect to separation, they selected a two-column system: a long (150-cm) column for the separation of the acidic and neutral amino acids, and a separate, short (15-cm) column for the basic amino acids. Because the basic amino acids remained on the first column in the standard run, the column had to be regenerated after each use. To ensure the possibility of continuous operation, two long columns were included in the system: while a sample was analyzed on one, the other was regenerated. In this way, when one analysis cycle was finished on one column, the second was ready for the next sample. Meanwhile, improved sulfonated polystyrene resins became available; they further fractionated the commercial product to obtain a narrower particle size cut. This method, based upon hydraulic flotation, was developed by P.B. Hamilton at the A.I. DuPont Institute of the Nemours Foundation (Wilmington, Delaware) (34). This improved packing permitted the use of higher flow rates without any loss of resolution.
The analyzer contained the three columns in a thermostat. Reciprocal pumps maintained a constant flow of the various buffer solutions used as the eluent and for column regeneration. Other major components were photometric detectors used at 570 and 440 nm, connected to a multipen potentiometric recorder, and a vessel between the columns and the detectors in which column effluent was continuously mixed with a ninhydrin solution. In this way, the amino acids reacted with ninhydrin, forming colored compounds. The system also included other devices, such as deaerators, manifold, valves, and gauges. Figure 2 shows a simplified schematic of the system. The sample was added manually by pipette to the top of the column, but from then on operation was unattended. One full analysis of a protein hydrolysate took one day and a more complex physiological fluid sample took about two days.

Figure 2
A preliminary description of the system was given at the April 1956 meeting of the Federation of American Societies for Experimental Biology (FASEB), in Atlantic City, New Jersey (35). The constructed breadboard system was used in a number of investigations at the institute. Finally, on February 28, 1958, the Rockefeller Institute team submitted two papers to Analytical Chemistry, describing in detail the preparation of the column packing, the operation of the column, and providing a very detailed description of the apparatus and its components, even giving an engineering drawing of the photometer consisting of three units. These two papers, entitled "Chromatography of Amino Acids on Sulfonated Polystyrene Resins" (3) and "Automatic Recording Apparatus for Use in the Chromatography of Amino Acids" (4) were published in the July issue of the journal. They represent the start of automated amino acid analysis.
Production of the Amino Acid Analyzer
Although the two papers provided a detailed description of the instrument constructed in the workshops of the Institute, probably only a few large laboratories would have been able to construct it. The Rockefeller Institute team realized this, and they turned to the Spinco Division of Beckman, with which they already had a good relationship, to transfer the design to production.
Specialized Instruments Company ("Spinco") was founded in 1946 by M.C. Hanafin and E.G. Pickels to commercialize the analytical ultracentrifuge developed at Rockefeller Institute under the direction of Dr. Pickels, constructed originally to aid the isolation of pure polio virus (36). On December 30, 1954, Beckman Instruments acquired the company that from then on, operated as the Spinco Division of Beckman.
Spinco built the first prototype of the amino acid analyzer — the so-called model MS (for Moore and Stein) — in the spring of 1958 (before the two papers were actually published), but originally, it had many of the usual problems associated with the transfer of a complicated design from research to production. At that time, Darrel Spackman left Rockefeller Institute and joined Spinco. He remained with the company for over three years and was involved in the improvements of the instrument; in 1962 he moved to the University of Washington (Seattle, Washington) and from then on, had academic affiliations. With Dr. Spackman's help, the instrument was soon "debugged" and from then on, the production of the model 120 Amino Acid Analyzer (its final designation) proceeded smoothly (Figure 3). Spinco also maintained a continuing contact with Dr. Moore, who regularly visited them (36).

Figure 3
In the subsequent years, the technique and the ion-exchange resins were improved further, permitting the use of a single column (37) and significant reduction of the analysis time. Spinco introduced the Model 120B in 1963 and the model 120C in 1966. Figure 4 shows a typical chromatogram from this period: a complex physiological sample now could be analyzed in 11 h instead of the two days that were required originally in 1958. For a simpler protein hydrolysate, the analysis time could be reduced to 4 h or even shorter. (In Figures 4 and 5, we do not detail the analytical conditions.)
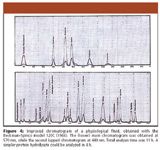
Figure 4
Spinco introduced additional models of the amino acid analyzer. Soon other instrument companies also entered the field, most notably Hitachi (Japan) whose amino acid analyzer was based primarily upon the investigations of H. Hatano (1924–1998), a professor at Kyoto University (Kyoto, Japan).
To illustrate the present status of amino acid analysis by ion-exchange chromatography, Figure 5 shows a typical chromatogram of a complex physiological fluid sample, with a total analysis time of 2 h. This chromatogram was obtained at the Chemical Laboratories of Missouri State Experiment Station at the University of Missouri (Columbia, Missouri). The laboratories have 10 Hitachi model L8800 fully automated amino acid analyzers in operation and handle about 1300 samples each month. Depending upon the sample complexity, each run takes 10 min to about 2.5 h. These data illustrate how advanced automated amino acid analysis has become.

Figure 5
Other Methods
Today, ion-exchange chromatography is not the only method used for the determination of amino acids: it is complemented by gas chromatography (GC), LC, and capillary electrophoresis (CE). To conclude our story, a few words about these developments are given here.
The use of GC for amino acid analysis became possible by the development of methods to prepare stable volatile derivatives, most notably the N-trifluoroacetyl n-butyl esters. At the University of Missouri, Gehrke and associates, in a decade of painstaking investigations, established the optimum methods for sample treatment and derivative formation, and for the optimum GC conditions permitting routine analysis (38,39). This work culminated in their investigation of lunar samples from Apollo 11 through 17 missions, for the possible presence of amino acids (40,41).
In LC, the key step in sample treatment involved the preparation of less polar derivatives permitting the use of reversed-phase chromatography and enhancing the possibilities of UV, fluorescence, and mass spectrometric (MS) detection. More recently, methods for the LC analysis of underivatized amino acids, with MS detection, also have been developed. Today, automated LC systems for amino acid analysis are available from a number of instrument companies.
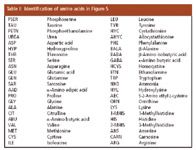
Table I: Identification of amino acids in Figure 5
The use of CE for amino acid analysis is relatively new and is still growing. CE is very tolerant to biological fluids and less or even no sample treatment is necessary. UV absorbance, fluorescence, and electrochemical detection as well as combination with MS is used.
The literature of amino acid analysis is vast, and we refer here only to a couple of useful recent review articles (42,43), as well as to two general monographs dealing with the state of art of the various analytical techniques (44,45).
With their pioneering work in the 1950s, Moore and Stein opened up an entirely new field for biochemists and it rightly can be considered as one of the great milestones in the century-old evolution of chromatography.
Acknowledgments
The activities of Moore and Stein leading to the amino acid analyzer have been documented in numerous literature sources, such as their autobiography (46), their Nobel lecture and the associated material (47), as well as the recollections of C.H.W. Hirs (32) and M.J. Gordon (36), and our report is based upon these sources. We would like to express our particular appreciation to Ms. Pat Ashton of the Heritage Exhibit at Beckman-Coulter, Fullerton, California, for information on Spinco and their activities related to the amino acid analyzer, and for the photos used in Figures 3 and 4. Dr. Thomas P. Mawhinney, Missouri State Experiment Station, kindly provided us the chromatogram shown in Figure 5. We also should note the help of Dr. Robert L. Wixom, Columbia, Missouri, concerning information on early amino acid research, and of Ms. Rebecca Graves, education services librarian at the Health Sciences Library, University of Missouri at Columbia, in literature search.
References to publications of Moore and Stein and their associates are given here to document the progress in their work leading to the amino acid analyzer. It was not our intention to present an exhaustive bibliography of their publications or to provide a review of the work of other scientists who contributed to the investigations of amino acids and proteins and to the development of analytical methods for amino acid analysis.
Charles W. Gehrke is professor emeritus of biochemistry at the University of Missouri, Columbia. He was associated for 37 years with the school and was also the head of the Missouri State Experiment Station Chemical Laboratories. He was a pioneer in the development of amino acid analysis by GC and has served as a co-investigator with Dr. Ponnamperuma of NASA for life molecules in the Moon rocks brought back by Apollo 11–17 spacecrafts. He is the recipient of numerous awards, among them the National Awards in Chromatography and in Separations Science of the American Chemical Society.
Leslie S. Ettre From 1988 to 2004, "Milestones in Chromatography" editor Leslie S. Ettre was associated with the Chemical Engineering Department of Yale University (New Haven, Connecticut), first as an adjunct professor and then as a research fellow. Previously, he had been with the Perkin-Elmer Corporation for 30 years. He is currently a member of LCGC's editorial advisory board.
References
(1) L.S. Ettre, LCGC 23(8), 752–761 (2005).
(2) L.S. Ettre, LCGC 23(5), 486–495 (2005).
(3) S. Moore, D.H. Spackman, and W.H. Stein, Anal. Chem. 30, 1185–1190 (1958).
(4) D.H. Spackman, W.H. Stein, and S. Moore, Anal. Chem. 30, 1190–1206 (1958).
(5) B. Helferich, in Great Chemists, E. Farber, Ed. (Interscience, New York, 1961), pp. 981–995.
(6) J.P. Greenstein and M. Winitz, Eds., Chemistry of the Amino Acids, Vol 2 (Wiley, New York, 1961), pp. 1299–1365.
(7) A.J.P. Martin and R.L.M. Synge, Biochem. J. 35, 1358–1368 (1941).
(8) R. Consden, A.H. Gordon and A.J.P. Martin, Biochem. J. 38, 224–232 (1944).
(9) S.R. Elsden and R.L.M. Synge, Biochem. J. Proc. 38, (1944).
(10) R.L.M. Synge, Biochem. J. 38, 285–294 (1944).
(11) R.L.M. Synge, Biochem. J. 39, 363–365 (1945).
(12) R.L.M. Synge, in Nobel Lectures Including Presentation Speeches and Laureates' Biographies — Chemistry 1942–1962 (Elsevier, Amsterdam, 1964), pp. 374–387.
(13) F. Sanger, in Nobel Lectures Including Presentation Speeches and Laureates' Biographies — Chemistry 1942–1962 (Elsevier, Amsterdam, 1964), pp. 544–556.
(14) T.I. Taylor and H.C. Urey, J. Chem. Phys. 8, 429–438 (1938).
(15) B.A. Adams and E.L. Holmes, J. Chem. Soc. Ind. (London) 547, 1 (1935).
(16) O. Samuelson, Z. Anal. Chem. 116, 328 (1939).
(17) O. Samuelson, Svensk. Kem. Tidskr. 51, 195–206 (1930).
(18) W.C. Johnson, L.L. Quill, and F. Daniels, Chem. Eng. News 25, 2494 (1947).
(19) L.S. Ettre, LCGC 17(12), 1104–1109 (1999).
(20) M. Bergmann and W.H. Stein, J. Biol. Chem. 128, 217–232 (1939).
(21) S. Moore, W.H. Stein and M. Bergmann, Chem. Rev. 30, 423–432 (1942).
(22) S. Moore and W.H. Stein, J. Biol. Chem. 150, 113–130 (1943).
(23) S. Moore, in Biographical Memoirs of the National Academy of Sciences (National Academy Press, Washington, DC, 1986), Vol. 56, 415–439.
(24) S. Moore and W.H. Stein, Ann. N.Y. Acad. Sci. 49, 265–278 (1948).
(25) W.H. Stein and S. Moore, J. Biol. Chem. 176, 337–365 (1948).
(26) S. Moore and W.H. Stein, J. Biol. Chem. 176, 367–388 (1948).
(27) S. Moore and W.H. Stein, J. Biol. Chem. 178, 53–77 (1949).
(28) W.H. Stein and S. Moore, J. Biol. Chem. 178, 79–91 (1949).
(29) W.E. Cohn, J. Biol. Chem. 186, 77–84 (1950).
(30) W.E. Cohn, J. Amer. Chem. Soc. 72, 1471–1478 (1950).
(31) S.M. Partridge, Biochem. J. 44, 521–527 (1949).
(32) C.H.W. Hirs, in The Beckman Symposium on Biomedical Instrumentation, C.L. Moberg, Ed. (Rockefeller University, New York, 1986) pp. 67–73.
(33) S. Moore and W.H. Stein, J. Biol. Chem. 192, 663–681 (1951).
(34) P.B. Hamilton, Anal. Chem. 30, 914–919 (1958).
(35) D.H. Spackman, W.H. Stein and S. Moore, Federation Proc. 15, 358 (1956).
(36) M.J. Gordon, in The Beckman Symposium on Biomedical Instrumentation, C.L. Moberg, Ed. (Rockefeller University, New York, 1986), pp. 31–35.
(37) P.B. Hamilton, Anal. Chem. 45, 2055–2064 (1963).
(38) W.M. Lamkin and C.W. Gehrke, Anal. Chem. 37, 383–389 (1965).
(39) C.W. Gehrke and D.L. Stalling, Separ. Sci. 2, 101–138 (1967).
(40) C.W. Gehrke, R.W. Zumwalt, K.C. Kuo, C. Ponnamperuma and A. Shimoyama, Origin of Life 6, 541–550 (1975).
(41) C.W. Gehrke, in Chromatography: a Century of Discovery 1900–2000, C.W. Gehrke, R.L. Wixom, and E. Bayer, Eds. (Elsevier, Amsterdam, 2001) pp. 83–97.
(42) P. Husek and P. Simek, LCGC 19(9), 986–999 (2001).
(43) S. Oguri, J. Chromatogr. B. 747, 1–19 (2000).
(44) C.W. Gehrke, K.C. Kuo, and R.E. Zumwalt, Eds., Amino Acid Analysis by Gas Chromatography, Vol. I–III (CRC Press, Boca Raton, Florida, 1987).
(45) I. Molnar-Perl, Ed., Quantitation of Amino Acids and Amines by Chromatography (Elsevier, Amsterdam, 2005).
(46) S. Moore and W.H. Stein, in 75 Years of Chromatography — a Historical Dialogue, L.S. Ettre and A. Zlatkis, Eds. (Elsevier, Amsterdam, 1979), pp. 297–308.
(47) S. Moore and W.H. Stein, in Nobel Lectures Including Presentation Speeches and Laureates' Biographies — Chemistry 1971–1980, T. Frängsmyr and S. Forsén, Eds. (World Scientific Publishing Co., Singapore and River Edge, New Jersey, 1993), pp. 73–95.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.












