Determining High-molecular-weight Phthalates in Sediments using GC–APCI-ToF-MS
LCGC Europe
A novel and sensitive approach for analysing high-molecular-weight phthalates is described.
The environmental analysis of phthalates is established and widespread with most classical methods based on gas chromatography mass spectrometry (GC–MS). Attention has recently turned to the analysis of di-isononylphthalate (DiNP) and di-isodecylphthalate (DiDP) but their analysis by GC results in an unresolved cluster of peaks that all predominantly fragment to the phthalic anhydride ion at m/z = 149. Consequently, quantification of DiNP and DiDP using the most abundant ion is impossible and the same sensitivity as for the single isomer phthalates cannot be reached. GC combined with atmospheric pressure chemical ionization (APCI) was used to analyse the high-molecular-weight phthalates. DiNP and DiDP were successfully ionized in proton transfer mode yielding spectra that contained the molecular ion as the base peak. The method was also applied to measure DiNP and DiDP in a sediment extract at relevant levels for environment samples.
The analysis of phthalates in environmental samples is an important application because some phthalates are considered to be potential endocrine-disrupting chemicals (EDCs).1 Most classical environmental methods focus on the analysis of the dialkyl esters of phthalic acid ranging from dimethylphthalate (DMP) to dioctylphthalate (DOP),2 among which di-isobutylphthalate (DiBP), dibutylphthalate (DBP) and bis(2-ethylhexyl)phthalate (DEHP) are the most important single isomer phthalates found in environmental samples.3,4 These solutes are single isomer compounds and relatively easy to analyse by GC–MS because only one peak for each compound is obtained by chromatographic analysis.5

Using electron impact (EI) ionization, the phthalic acid diesters strongly fragment, the most abundant ion being a phthalic anhydride ion at m/z = 149, common for all saturated alkyl phthalates (except DMP). This ion can be used for trace analysis in selected ion recording (SIR) mode, provided that the target solutes are well separated by GC.5
More recently, attention has been paid to the analysis of DiNP and DiDP. These compounds are synthesized from phthalic acid and a mixture of C9- or C10-alcohols, respectively. Consequently, the GC analysis of these solutes results in a cluster of peaks corresponding to different branched isomers.
In the case of DiNP and DiDP determination, the C9-isomers present in commercial DiNP samples partially overlap with the C10-isomers present in DiDP. No column or temperature programme conditions could be found to completely separate C9- from C10-isomers, making quantification of DiNP and DiDP using the most abundant ion at m/z =149 impossible. To differentiate DiNP and DiDP, another ion should be measured. This can be done using ions 293 and 307 respectively, corresponding to the M-alkyl ion.5 These ions are, however, relatively low in abundance and consequently the same sensitivity as for the single isomer phthalates cannot be reached.
Possible solutions are offered by the use of soft ionization techniques, such as chemical ionization (using ammonia), or by LC–MS. Recently, the combination of GC with atmospheric pressure chemical ionization (APCI) became commercially available6 and this technique was tested for the analysis of the high-molecular-weight phthalates. The method was also applied to measure DiNP and DiDP in a sediment extract from The Netherlands.
Experimental
Reference samples of DiNP (C26H42O4) and DiDP (C28H46O4) were obtained from BASF (Ludwigshafen, Germany). Individual stock solutions at 1000 ppm were prepared in cyclohexane. Calibration solutions containing DiNP and DiDP at 1–10 ppm level were prepared by dilution in cyclohexane.
A sediment sample, obtained during an environmental study,4 was dried by lyophylization and a 10 g sample was extracted with 50 mL cyclohexane in an ultrasonic bath. The extract was concentrated to 10 mL in a Zymark Turbovap system (Caliper Life Sciences, Teralfene, Belgium).
Analysis was performed on an Agilent 7890 GC (Palo Alto, California, USA) coupled to a Waters Xevo QTOF MS system (Waters. Manchester, UK). Sample introduction was performed using a CTC GC-Pal autosampler (Basel, Switzerland) via a split/splitless injector. The Xevo QTOF was operated in GC–MS mode using an atmospheric pressure GC (APGC) source (Waters, Manchester, UK). Water was continually bled into the source during acquisition to promote proton transfer conditions.
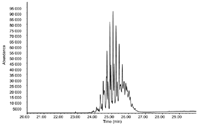
Figure 1: GCâEI-MS total ion chromatogram (TIC) obtained for a DiNP/DiDP mixture (10 ppm).
The following chromatographic conditions were used:
Injection: 1 μL, splitless, 300 °C, 1.05 min purge delay
Column: 30 m × 0.25 mm i.d. × 0.25 μm DB-5MS (J & W Scientific, Folsom, California, USA)
Carrier: Helium, 2 mL/min constant flow
Oven: 50 °C – 1 min – 20 °C/min – 320 °C – 2.5 min
The instrument was tuned and calibrated using perfluorotripentylamine (FC 70) before acquisition, so that the resolution of the instrument was greater than 10000 full width half maximum (FWHM). Exact mass spectra were obtained (5 Hz) using a single-point lock mass (column bleed, m/z = 281.0517) infused into the source continuously during the temperature programme.
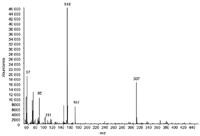
Figure 2: EI-MS spectrum of di-isodecyl phthalate (C28H46O4, MW = 446).
The following MS conditions were used:
Corona current: 4.0 μA
Sampling cone: 10 V
Source temperature: 140 °C
Cone gas: Nitrogen at 30 L/hr
Source auxiliary gas: Nitrogen at 300 L/hr
Make-up gas: Nitrogen at 300 mL/min
GC interface temperature: 310 °C
Results and Discussion
GC–EI-MS (Single quadrupole system): A typical total ion chromatogram (TIC) chromatogram obtained for the 10 ppm standard mixture of DiNP and DiDP using electron impact ionization (performed on a single quadrupole instrument) is shown in Figure 1. A complex cluster of DiNP and DiDP isomers is observed. The spectrum taken at 25.6 min, corresponding to the most abundant DiDP isomer is given in Figure 2. The molecular ion at m/z = 446 (C28H46O4) cannot be detected and the main ions are at m/z =149 (phthalic anhydride, C8H5O3+) and 307 (M-C10H19).
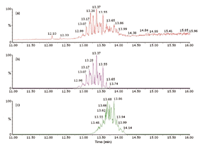
Figure 3: GCâAPCI-ToF-MS analysis of a 10 ppm standard mixture containing DiNP and DiDP. (a) TIC;(b) XIC at 419.316 (DiNP); (c) XIC at 447.347 (DiDP).
Differentiation of the two high-molecular-weight phthalates can only be completed by monitoring the ions at 293 (DiNP) and 307 (DiDP). However, due to the low abundance of these ions compared to the base peak, lower sensitivity is obtained, and, moreover, confirmation by a second ion is not possible.
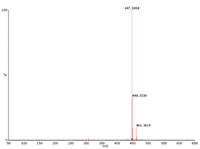
Figure 4: Proton-transfer APCI spectrum for DiDP (m/z = 447.3494 = [C28H46O4 + H]+).
GC–APCI-ToF: The analysis of the 10 ppm standard mixture was also performed using the conditions described in the experimental section. The TIC and the extracted ion chromatograms (XIC) at m/z = 419.316 (DiNP) and 447.347 (DiDP) are shown in Figure 3. The mass spectrum at 13.7 min (DiDP fraction) is shown in Figure 4. DiNP and DiDP give strong pseudo-molecular ions, [M + H]+ with no fragmentation compared with EI ionization. The main ion in the spectrum of DiDP is at m/z = 447.3494, a deviation of 4.5 ppm from the theoretical value for [C28H46O4 + H]+ . The extracted ion chromatograms for DiNP and DiDP, included in Figure 3, show that partial chromatographic separation is possible between these two compounds. However, the XICs are needed to allow quantification. The exact mass detection enhances selectivity and allows confirmation of the presence of the individual solutes. Interestingly, the spectrum for DiDP shown in Figure 4, also contains m/z = 461.3615, – 3.5 ppm from the calculated mass of C29H49O4. This can probably be explained by the presence of mixed undecyl-decyl phthalates in the DiDP standard. If traces of C11-alcohols were present in the C10-alcohols used in DiDP synthesis, this would result in these impurities. Next, a sediment extract was also analysed by GC–APCI-ToF-MS. The extracted ion chromatograms at 419.316 (DiNP) and 447.347 (DiDP) are given in Figure 5. Both solutes can easily be detected. It is, however, interesting to observe that the isomer distribution of DiNP and DiDP detected in the sediment sample are different from the standard mixture. Moreover, by using XICs at 433.332 and 461.363, additional phthalate solutes are detected. The masses correspond to C27H44O4 and C29H48O4 respectively, and are probably mixed C9/C10 and C10/C11 phthalates. At the end of each phthalate elution window, a single abundant peak is detected (for instance at 13.74 min for DiNP and at 14.41 min for DiDP). These probably correspond to the linear di-C9 and di-C10 phthalates, respectively. The calculated concentrations of DiNP and DiDP in this sediment sample are 0.2 and 2 ppm respectively.
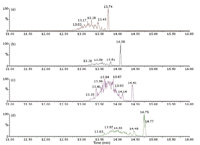
Figure 5: Detection of highâmolecularâweight phthalates in sediment sample by GCâAPCI-ToF-MS (a) XIC at 419.316 (DiNP); (b) XIC at 433.332 (C9/C10); (c) XIC at 447.347 (DiDP); (d) XIC at 461.363 (C10/C11).
Conclusions
DiNP and DiDP were successfully ionized in proton transfer APCI, yielding spectra that contained the molecular ion as the base peak. These high-molecular-weight phthalates could be detected below 1 ppm in sediment samples using APCI by extracting exact mass chromatograms. At least two additional phthalate moieties were also identified in the extracted sediment sample using exact mass chromatograms. The calculated formulae C27H44O4 and C29H48O4, suggest mixed C9/C10 and C10/C11 phthalates, respectively. Good exact mass calculation was obtained for all solutes, with mass errors less than 5 ppm. GC–APCI- ToF-MS therefore offers interesting perspectives for detailed characterization of high-molecularweight phthalates and for their sensitive and selective detection in environmental samples. The APGC source used allows all of the acquisition modes of tandem quadrupole and QToF spectrometers to be used with a GC inlet combined with a soft ionization mechanism.
Frank David is R&D Manager at RIC, Kortrijk, Belgium and visiting professor at Ghent University, Belgium.
Pat Sandra is director of the Research Institute for Chromatography (RIC), Kortrijk, Belgium, professor in Separation Science at Ghent University, Belgium and director of the Pfizer Analytical Research Centre-UGent, Belgium.
Peter Hancock is Technical Manager in the Chemical Analysis Business Operations team working for Waters Corporation, Manchester, UK, where he and his team are actively involved in developing analytical methods on new MS technologies in application areas such as food and environmental testing and chemical materials.
References
1. S. Jobling et al., Environ Health Perspect., 103(6), 582–587 (1995).
2. DIN EN ISO 18856.
3. H. Fromme et al., Water Research, 36(6), 1429–1438 (2002).
4. W.J.G.M. Peijnenburg and J. Struijs, Ecotoxicology and Environmental Safety, 63(2), 204–215 (2006).
5. F. David et al., in Phthalate Esters, C.A. Staples (Ed), Springer, Berlin, 2003, pp. 9–56.
6. K. Worrall and P. Silcock, The Column, 6(10), 3–6, (2010).

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.












