Determination of Stilbenes in Blueberries
LCGC North America
There is growing evidence that blueberries have powerful disease-fighting properties. Here the authors explore the research being done in this area, describing the use of a magnetic seector GC-MS SIM method to determine the presence of phenolic antioxidants in blueberries.
There is growing evidence that blueberries have powerful disease-fighting properties. The diminutive, dusky blue fruit recently has caught the attention of researchers and is now attributed with such human health benefits as high antioxidant activity (1), antiaging properties (2), the ability to improve eyesight (3) and brain function (4), and lipid-lowering properties (5).

Recent studies have correlated the high antioxidant activity of blueberries with their total phenolic content. Phenolics include a group of compounds called stilbenes. This article discusses a study to determine the presence of the stilbenes resveratrol, pterostilbene, and piceatannol (Figure 1) in blueberries by gas chromatography–mass spectrometry (GC–MS) in a selected ion monitoring (SIM) mode.
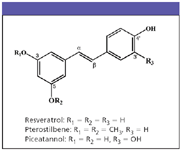
Figure 1: Structures of resveratrol, pterostilbene, and piceatannol. Reprinted with permission from the Journal of Agricultural and Food Chemistry. Copyright 2004 American Chemical Society.
Experimental
Vaccinium berries growing wild were collected at various sites, while other samples were grown commercially. Samples (approximately 50-g fresh weight) were collected randomly and placed in chilled containers pending transport to the United States Department of Agriculture (USDA), Agricultural Research Service (ARS), Small Fruits Research Station (SFRS), Poplarville, Mississippi. The berries were freeze-dried and sent to the Natural Products Utilization Research Unit, Oxford, Mississippi. The samples from Canada were sent as extracts that had been passed through a C18 column. All samples were stored for about six months in a cold room maintained at 4 °C until extraction and analysis.
Extraction of berries from U.S. locations: Berries were extracted using an ASE apparatus (Dionex Corporation, Sunnyvale, California). A 1-g amount of lyophilized berry was mixed with about 10 g of purified sand and loaded in the extraction cartridge. Purified sand was further added to pack the extraction cartridge fully. Extraction was performed with the following parameters: heat, 5 min; static, 10 min; flush volume, 100 mL; purge, 90 s; pressure, 1000 psi; temperature, 40 °C; extraction solvent, 40:40:20:0.1 (v/v/v/v) methanol–acetone–water–acetic acid (four cycles). The extract was concentrated under vacuum using a rotary evaporator to remove the organic solvents. The resulting aqueous solution was extracted with 1 mL of ethyl acetate (three times). The combined ethyl acetate extract was evaporated to dryness under a stream of nitrogen.
Extraction of berries from Canada: A 50-g amount of frozen berries was mixed with three volumes of 40:40:20:0.1 (v/v/v/v) methanol–acetone–water–formic acid, kept for 30 min, then ground in a Virtis homogenizer (The Virtis Co., Gardiner, New York) in a stainless steel flask for 2 min. The extract was filtered through an 11-cm diameter G6 glass fiber filter, and the residue was extracted a second time with 60 mL of extraction solvent, kept for 30 min, then ground for 2 min and filtered. The filtrates were combined into a tared round-bottom flask, and the organic solvents were removed under vacuum. The remaining aqueous portion was freeze-dried, suspended in approximately 10 mL of water, and loaded on a 10-g C18 column (Waters Scientific, Mississauga, Ontario, Canada). The retained components were eluted with about 50 mL of 40:40:20:0.1 (v/v/v/v) methanol–acetone–water–formic acid. The organic components of the solvent were removed under vacuum, and the aqueous portion was freeze-dried.
Analysis of resveratrol, pterostilbene, and piceatannol: A 1-mg amount of sample was derivatized with 100 μL of a mixture of 3.5 : 1 : 0.5 (v/v/v) bis-(trimethylsilyl)trifluoroacetamide–dimethyl formamide–methanol in a 2-mL GC vial. The vial was capped and heated at 70 °C for 1 h. After the vial cooled to room temperature, 2 μL was injected and analyzed by GC–MS on a JEOL GCMate II benchtop magnetic sector MS system (JEOL USA, Inc., Peabody, Massachusetts). The GC temperature program was as follows: initial temperature, 150 °C; increased to 260 °C at a rate of 25 °C/min; then increased to 270 °C at a rate of 1 °C/min; increased to 320 °C at a rate of 60 °C/min; and held at this temperature for 2 min. The GC capillary column was a 3 m × 0.25 mm, 0.25-μm, ZB-50 (Phenomenex, Torrance, California). The carrier gas was ultrahigh purity helium at a 1-mL/min flow rate. The inlet (splitless), GC interface, and ion chamber temperatures were 250, 250, and 230 °C, respectively.
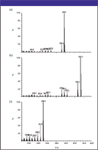
Figure 2: Mass chromatograms of: (a) resveratrol, (b) piceatannol, and (c) pterostilbene showing major fragmentations. Reprinted with permission from the Journal of Agricultural and Food Chemistry. Copyright 2004 American Chemical Society..
The analysis of resveratrol, piceatannol, and pterostilbene was performed using SIM mode (retention times 8.7, 9.5, and 10.2 min, respectively (Figure 2). Resveratrol was monitored at m/z 444 (428, 370, and 354 as qualifier ions). Pterostilbene was monitored at m/z 328 (312, 296, and 280 as qualifier ions). The qualifier ions were selected as being the most abundant ions in the mass chromatogram of the respective standard samples. Piceatannol was monitored at m/z 532 (516, 443, and 428 as qualifier ions) (Figure 3). Quantitation was done using external standards of commercial samples of resveratrol and piceatannol and a synthetic sample of pterostilbene. The limits of detection for resveratrol, piceatannol, and pterostilbene were 1, 21, and 18 ng/g dry sample, respectively, and limits of quantitation were 23, 69, and 61 ng/g dry sample, respectively.
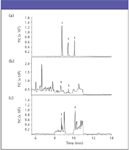
Figure 3: Gas chromatogram of (a) mixture of standard samples of resveratrol (1), piceatannol (3), and pterostilbene (2) with retention times of 8.7, 9.5, and 10.2 min, respectively; (b) extract of V. corymbosum L. (highbush blueberry) cv. Bluecrop (sustainable) from Corvallis, Oregon; and (c) extract of V. ashei Reade (rabbiteye blueberry) cv. Tifblue from Lamar Co., Mississippi. Reprinted with permission from the Journal of Agricultural and Food Chemistry. Copyright 2004 American Chemical Society.
Results and Discussion
Samples from four locations in North America, representing selections and cultivars of 10 species of Vaccinium fruits were analyzed using a GC–MS SIM technique. By monitoring only masses that are characteristic of the target compounds, this method allowed for a more selective detection and more accurate determination of the stilbenes in the samples. Variable amounts of resveratrol, pterostilbene, and piceatannol were found in extracts of whole berries of the Vaccinium species randomly collected from Canada and the states of Mississippi, North Carolina, and Oregon.
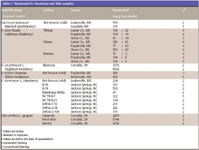
Table I: Resveratrol in Vaccinium and Vitis samples
Resveratrol was found in all species of the Vaccinium berries that were analyzed in this study, varying in levels from 7 ng/g to as high as 5884 ng/g dry sample (Tables I and II). The highest level compares with content found in grapes, which ranged between 2475 and 6471 ng/g dry sample. Higher levels of resveratrol were found in the berries from Canada, as compared with those from North Carolina, Mississippi, and Oregon. Lingonberry, obtained from Nova Scotia, was found to have the highest content of resveratrol, almost the same as that found in grapes. Although a comparative extraction study was not conducted, not being the objective of this study, differences in extraction procedures can account for the higher resveratrol content in samples from Canada.
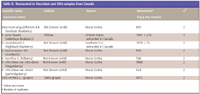
Table II: Resveratrol in Vaccinium and Vitis samples from Canada
The occurrence of resveratrol in Vaccinium berries is of no surprise, as resveratrol seems to be widespread in the plant kingdom. The variability of the resveratrol content of Vaccinium berries in this study could be attributed to biotic and abiotic stresses such as UV light and Botrytis cinerea infection (6) and with time of harvest, environmental and climatic conditions, and plant developmental stage (7–10). It is unlikely that storage conditions played a factor in the variation of levels of resveratrol found in this study. It has been reported that resveratrol content in grape pomace and skins stored over five months in the dark at ambient temperatures varying between 5 and 18 °C showed no change (11).
Pterostilbene was found in three samples: two cultivars of V. ashei (rabbiteye blueberry from Mississippi) and in V. stamineum (deerberry from North Carolina) at levels of 99–520 ng/g dry sample. Piceatannol was found in deerberry, highbush blueberry, and blackberry at levels of 138–422 ng/g dry sample (Table III).
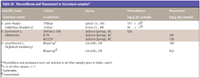
Table III: Pterostilbene and Piceatannol in Vaccinium samplesa
In grapes, pterostilbene and piceatannol generally are found in very minute quantities and might be completely absent. These two compounds were not found in the grape samples analyzed in this study, even with the sensitive GC–MS SIM method used. Pterostilbene has been detected in fungus-infected grapes at levels of 0.2–4.7 μg/g fresh weight of skin (12) and also in healthy grape berries of the Gamay and Pinot Noir variety at levels of 14–74 ng/g and 120–530 ng/g fresh berries, respectively (13). Pterostilbene is the only known stilbene in the genus Pterocarpus and was found to be one of two antidiabetic compounds isolated from the heartwood of Pterocarpus marsupium (14). Piceatannol has been found in significant amounts (0.052 μg/g fresh wt) in Vitis vinifera cv. Cabernet Sauvignon (15). Like resveratrol, the piceatannol content in grapes is increased by UV-C irradiation. Piceatannol appears to be present in diverse plant groups: Maclura pomifera (16), Senna skinner (17)i, Rheum spp. (18), and Cassia spp. (19).
It is of great value to have shown that some small fruits of Vaccinium such as blueberries contain these stilbenes that are known to possess health beneficial (such as cancer chemopreventive [20,21], antioxidant [22], and hypolipidemic [23]) properties. It has been hypothesized that additive and synergistic effects of complex mixtures of phytochemicals, instead of a single component, provide the health benefits derived from fruits and vegetables (24). The stilbenes in Vaccinium berries could add to or act synergistically with other phytochemicals in the berries to produce a more effective complement of protection against coronary heart disease and provide cancer chemoprevention.
Conclusion
The sensitive GC–MS SIM method employed in this study enabled the accurate determination of resveratrol, pterostilbene, and piceatannol that showed the presence in varying amounts of these compounds in Vaccinium berries. This finding adds value to these small fruits.
Disclaimer: Mention of trade names or company commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Dr. Agnes M. Rimando received the Ph.D. degree in pharmacognosy from the University of Illinois at Chicago in 1993. She is currently a Research Chemist with the USDA ARS, Natural Products Utilization Research Unit, Oxford, Mississippi. She is serving as the 2005 Vice-Chair of the American Chemical Society Agricultural and Food Chemistry Division.
Dr. Robert Cody received the Ph.D. degree in analytical chemistry from Purdue University, West Lafayette, Indiana, in 1982. He is currently a Product Manager of mass spectrometry products at JEOL USA, Inc., Peabody, Massachusetts, a member of the American Chemical Society (Analytical Division) and the American Society for Mass Spectrometry.
References
(1) I. Scibisz et al., Zywnosc 10(2, Supl.), 159–166 (2003).
(2) J.A. Joseph and B. Shukitt-Hale, NATO Science Series, Series I: Life and Behavioral Sciences 358, 170–181 (2004).
(3) T. Tamada, Food Style 21(6), 55–59 (2002).
(4) K.A. Youdim et al., Nutr. Neurosci. 3, 383–397 (2000).
(5) A. Cignarella et al., Thrombosis Res. 84, 311–322 (1996).
(6) R. Preisig-Mulleret et al., Plant Molec. Biol. 39, 221–229 (1999).
(7) A.J. Bais et al., Aus. J. Plant Physiol. 27, 425–433 (2000).
(8) R. Eder et al., Mitteilungen Klosterneuburg 51, 64–78 (2001).
(9) M. Keller et al., Acta Hort. 514, 275–286 (2000).
(10) J.M. Moriarty et al., Vitis 40, 43–44 (2001).
(11) A.A.E. Bertelli et al.; Drugs Exper. Clin. Res. 24, 207–211 (1998).
(12) M. Adrian et al., J. Agric. Food Chem. 48, 6103–6105 (2000).
(13) R. Pezet and V. Pont, Plant Physiol. Biochem. 26, 603–607 (1988).
(14) M. Manickam, M. Ramanathan, M.A.F. Jahromi, J.P.N Chansouria, and A.B. Ray. J. Nat. Prod. 60, 609–610 (1997).
(15) L. Bavaresco et al., Vitis 41, 133–136 (2002).
(16) S.C. Wang et al., Wood Fiber Sci. 15, 290–301 (1983).
(17) D. Arrieta Baez et al., Nat. Prod. Lett. 13, 223–228 (1999).
(18) P. Suhag et al., J. Med. Arom. Plant Sci. 21, 1026 (1999).
(19) R.H. Liu, Appl. Biotech. Food Sci. Policy 1, 39–46 (2003).
(20) M. Jang et al., Science 275, 218–220 (1997).
(21) A.M. Rimando et al., J. Agric. Food Chem. 50, 3453–3457 (2002).
(22) L.A. Stivala et al., J. Biol. Chem. 276, 22586–22594 (2001).
(23) A.M. Rimando et al., Proc. 228th Nat. Meeting Amer. Chemical Soc., Philadelphia, Pennsylvania. AGFD 085 (2004).
(24) R.H Liu. Appl. Biotech. Food Sci. Policy 1, 39–46 (2003).
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.











