Determination of Pharmaceuticals from Serum
The Application Notebook
This application note describes the determination of pharmaceuticals from serum using solid-phase extraction (SPE) with the hydrophilic-lipophilic balanced SPE phase CHROMABOND® HLB for analyte enrichment and for sample cleanup. The eluates from SPE are finally analyzed by HPLC–MS/MS on a NUCLEOSHELL® PFP core–shell phase.
This application note describes the determination of pharmaceuticals from serum using solid-phase extraction (SPE) with the hydrophilic-lipophilic balanced SPE phase CHROMABOND® HLB for analyte enrichment and for sample cleanup. The eluates from SPE are finally analyzed by HPLC–MS/MS on a NUCLEOSHELL® PFP core–shell phase.
Nowadays, people suffer from various diseases, and are prescribed many types of pharmaceuticals as part of their treatment, for example, anesthetics, antibiotics, anticholinergics, anticonvulsants. In order for the treatment to be successful, it is necessary to keep controlling the levels of the pharmaceuticals to provide an accurate dosage. This has led to an increasing demand for the development of accurate and sensitive analytical methods to analyze the pharmaceuticals from serum to protect human health.
Solid-Phase Extraction (1)
SPE column: CHROMABOND® HLB, 1 mL, 30 mg, MACHEREYâNAGEL REF 730921
Column conditioning: 1 mL methanol, then 1 mL water
Sample application: 1 mL spiked serum sample is passed through the column by vacuum.
Washing: 1 mL water
Drying: 10 min with vacuum
Elution: 2 mL methanol
Eluent exchange: Eluate is evaporated to dryness at 40 °C under a stream of nitrogen and reconstituted in 1 mL 95:5 (v/v)
water–acetonitrile
Subsequent Analysis: HPLC–MS/MS (2)
HPLC column: EC 50/2 NUCLEOSHELL® PFP, 2.7 μm, MACHEREYâNAGEL REF 763532.20
Eluent A: 0.1% formic acid in water
Eluent B: 0.1% formic acid in acetonitrile
Gradient: 5–95% B in 7.5 min, 95% B for 1 min, 95–5% B in 0.5 min, 5% B for 5 min
Flow rate: 0.3 mL/min
Temperature: 30 °C
Injection volume: 5 µL
MS/MS detection: API 5500 (AB Sciex GmbH); ion source: ESI; positive ionization mode; scan type: selected reaction monitoring (SRM, for transitions see Table 1); detection window: 90 s; curtain gas: 40 psig; ion spray voltage: 5500 V; temperature: 500 °C; nebulizer gas: 45 psig; turbo gas: 45 psig; CAD: medium.
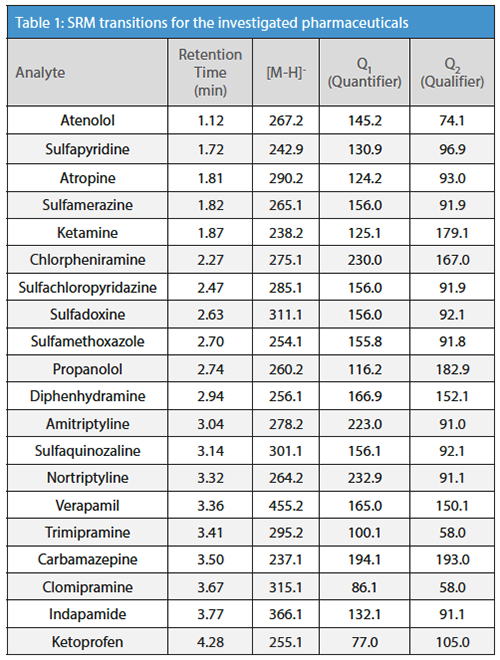
Results
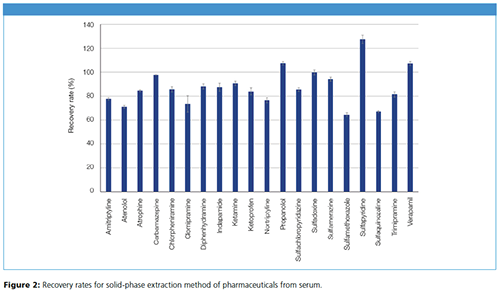
The recovery rates show that the determination of pharmaceuticals from serum could be carried out successfully (Figure 2). By using SPE with CHROMABOND® HLB, it was possible to recover nearly all pharmaceuticals from serum, with good reproducibility on average. Regarding the different types of pharmaceuticals, the average recovery rates were: for anesthetics 90.8%, antibiotics 94.4%, anticholinergics 84.8%, anticonvulsants 97.7%, antidepressants 77.4%, antihistamines 87.1%, anti-inflammatory drugs 84.1%, beta blockers 89.5%, calcium channel blockers 107.5%, and for diuretics 87.7%.
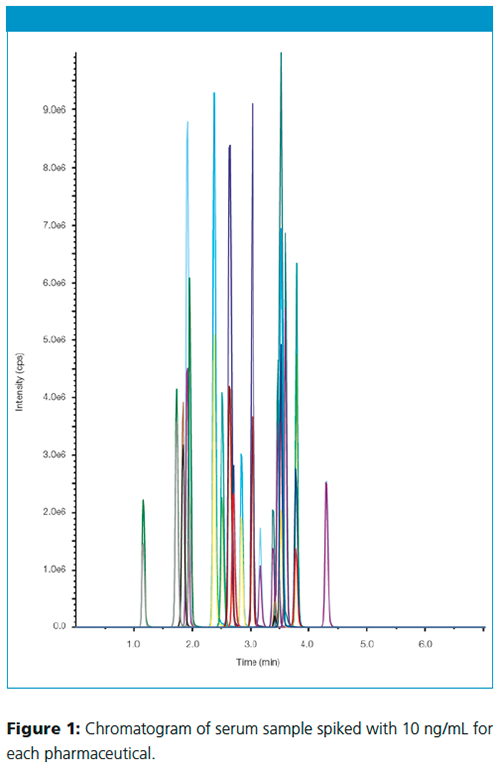
The identification and quantification of pharmaceuticals in the solid-phase extracts were performed using ESI-MS on an EC 50/2 NUCLEOSHELL® PFP column. The chromatogram in Figure 1 shows the results of solid-phase eluate spiked with 10 ng/mL serum for each pharmaceutical.
Conclusion
The presented application describes a quick and convenient method for the determination of pharmaceuticals from serum by SPE with a hydrophilic-lipophilic balanced phase, followed by HPLC–MS/MS analysis.
References
- Application No. 306510, MACHEREY-NAGEL, available from www.mn-net.com/apps
- Application No. 128200, MACHEREY-NAGEL, available from www.mn-net.com/apps

MACHEREY-NAGEL GmbH & Co. KG
Neumann-Neander-Str. 6 – 8, 52355 Düren, Germany
Tel.: +49 24 21 969 0 Fax: +49 24 21 969 199
E-mail:info@mn-net.com Website:www.mn-net.com
SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




