Detection of Doping Agents by LC–MS and LC–MS-MS
LCGC North America
In the last decade, research on the detection of all groups of doping agents has been investigated by LC-MS, and routine LC-MS screening applications are now available for almost all classes of doping agents.
Initially, the introduction of liquid chromatography–mass spectrometry (LC–MS) into the field of doping analysis focused primarily on substances that were difficult to detect by other techniques. The first application of LC–MS in antidoping analysis was the detection of the highly polar and thermally labile diuretics. Further research also revealed that good results for nonpolar doping agents can be achieved with LC–MS. Corticosteroids, for example, exhibit a sensitivity, selectivity, and specificity not reached by any other analytical technique. In the last decade, research on the detection of all groups of doping agents has been investigated by LC–MS and routine LC–MS screening applications are now available for almost all classes of doping agents. Improvements in MS technology with respect to scan speed, sensitivity, and polarity switching allows for the combined detection of different classes of doping agents. Low physiological levels in urine and plasma mean the detection of peptide hormones is still difficult by LC–MS. In general, it is predictable that the application of LC–MS in antidoping analysis will increase.
The drive to compete and win is as old as humankind. Throughout history, athletes have sought foods and potions to transform their bodies into powerful, well-tuned machines.
The Roman gladiators of Circus Maximus used stimulants mixed with alcohol to overcome fatigue and injuries, and Scandinavian warriors (the Berserkers) ate hallucinogenic mushrooms to gear up for battle (1). The first competitive athletes believed to be charged for doping were swimmers in Amsterdam in the 1860s. Doping controls started in 1967 after Tom Simpson died on the Mont Ventoux from a combination of exhaustion, amphetamines, and alcohol.
In contrast to forensic sciences, in which blood is the most important matrix, urine is preferred in doping analysis. The advantage of urine is the relatively large volume that can be collected when compared with blood. Analytes also are present in urine in higher concentrations than in blood.
In addition, urine collection is noninvasive, and its use takes into account the fact that top athletes are controlled several times during major events.
Before the introduction of liquid chromatography–mass spectrometry (LC–MS) in the field of doping analysis, MS information was acquired with capillary gas chromatography–mass spectrometry (GC–MS). Unfortunately, this technique is only suitable for volatile or thermostable compounds. Because many doping agents have polar functional groups in their structure, their volatility is limited and before GC–MS analysis, a derivatization step is required. Unfortunately, some derivatives are thermally labile and cannot be detected.
LC–MS analysis of these compounds does not require a derivatization step and in the 1980s, many efforts were made to interface liquid chromatography with mass spectrometry. At the beginning of the 1990s commercial LC–MS instrumentation became available in doping control laboratories and research focused primarily on substances for which GC–MS detection was difficult or impossible. In 1991, a first approach for the analysis of diuretics by LC–MS showed promising results (2). Despite these good results, technical limitations impeded its routine application. In the coming years, the quality of LC–MS instrumentation improved and concurrently, the purchase costs decreased. As a result, routine LC–MS detection methods became available in many doping control laboratories by the end of the 1990s. As a consequence, routine detection methods for several classes of doping substances were developed. These early LC–MS methods were reviewed in 2005 (3). The aim of this work is to describe the current state in the application of LC–MS in the field of human antidoping analysis. In this article. the classes of doping substances will be discussed as they appear on the WADA-prohibited list (4). An overview of common LC–MS detection strategies for doping substances is presented in Table I.
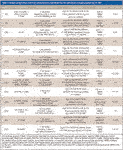
Table I: Sample preparation and mass spectrometric conditions for the detection of doping agents by LCâMS
Compounds Prohibited in and out of Competition
Anabolic steroids: Anabolic steroids are synthetic derivatives of the male hormone testosterone that are used by athletes to increase their strength. Although steroids have been banned since 1976, they remain the most detected doping substances by accredited laboratories (5). The detection of anabolic steroids in human urine normally is performed by GC–MS after hydrolysis and derivatization.
LC–MS opened the possibility of directly detecting anabolic steroids without derivatization. Unfortunately, LC–MS analysis of these analytes also presents several difficulties including limited ionization when using conventional atmospheric pressure interfaces electrospray ionization (ESI) and atmospheric chemical ionization (APCI). Due to this problem, the number of steroids included in LC–MS screening methods was originally limited to those substances for which the GC–MS detection was problematic. Three steroids (gestrinone, tetrahydrogestrinone [THG], and 39OH-stanozolol) have been included in a screening method for 28 doping agents (6), achieving limits of detections of 2 ng/mL for 39OH-stanozolol and 5 ng/mL for THG and gestrinone, respectively. Similar limits of detection (LODs) were obtained in our laboratory for the screening of these three anabolic steroids and 17α-trenbolone (7). In addition, the applicability of LC–MS for the identification of different stanozolol metabolites was pointed out.
A more detailed study on the detection of stanozolol metabolites has been described by Thevis and colleagues (8). In this article, a method for the detection of stanozolol and three metabolites is reported with LODs lower than 0.2 ng/mL after solid phase extraction.
Recently, ESI of anabolic steroids has been investigated in our laboratory (9), indicating that anabolic steroids containing a conjugated keto function or a nitrogen can be easily ionized as [M+H]+ while other steroids can be better ionized after the formation of adducts with mobile phase components such as ([M+NH4]+, [M+H+CH3OH]+ or [M+H+CH3CN-H2O]+). These ionization experiments were subsequently used to develop a comprehensive screening method for the detection of 34 anabolic steroids (10).
One of the main challenges of LC–MS in doping analysis is the detection of new and unknown designer steroids (11). Different approaches have been described. Thevis and colleagues (12) developed a method based upon precursor ion scan by selecting several ions typically appearing when the steroids have a common structure. This approach has been shown useful for analytes having a preselected structure. LC–MS also has shown its potential to detect unknown steroids as in the case of the new long-term metabolite of metandienone (13) or endogenous interferences, which can hamper the detection of exogenous anabolic steroids (14).
Peptide hormones and related substances: When LC–MS was introduced in the 1990s, doping laboratories focused on the implementation of this technique for the detection of small molecules (nonpeptide substances). Currently, these LC–MS methods are well optimized, and doping laboratories are investigating the LC–MS detection of peptide hormones in urine or blood.
Sensitivity is the major problem for the detection of peptide hormones. Peptide hormones generally are administered in low doses and consequently, the concentrations in blood and urine are fairly low (picrogam-per-milliliter or low nanogram-per-milliliter level). In general, they are detected by immunological techniques, but because scientific evidence obtained with MS is prefered, doping control laboratories are developing LC–MS methods to confirm the presence of peptides.
Insulin is used medically to treat some forms of diabetes mellitus. Insulin gained interest among athletes because it helps the cellular uptake of glucose and amino acids. Athletes suffering from diabetes can obtain a therapeutic use exemption (TUE). Endogenous insulin concentrations vary between 0.1 and 3.2 ng/mL. Although insulins have been on the prohibited list since 1999, the first doping-related LC–MS application was described in 2005 by Thevis and colleagues (15). Reversed-phase LC–ESI-MS-MS detection of the synthetic analogues Humalog, Novolog, and Lantus in plasma focused on the fivefold protonated molecules [M+5H]5+ after immunoaffinity clean-up (Table I). The detection limit of 0.5 ng/mL should be sufficient for antidoping control purposes.
Tetracosactide is a synthetic analogue of human adrenocorticotropin (ACTH). ACTH stimulates the release of cortisol. Because corticosteroids are on the prohibited list, this cortisol-releasing hormone also is prohibited. Recently, the detection of synacthen has been described by Thevis and colleagues (16). The plasma samples were purified using immunoaffinity chromatography and solid-phase extraction. The compounds were separated by reversed-phase LC. ESI-MS-MS detection was carried out in selected reaction monitoring. The developed method required 2 mL of plasma and allowed for an LOD of 100 fmol/mL (300 pg/mL).
Hemoglobin-based oxygen compounds are used as artificial oxygen carriers. They can be used in endurance sports to increase oxygen transport. An important compound of this group is Hemopure. Hemopure is derived from bovine hemoglobin. Bovine and human hemoglobins differ by 15% in amino acid sequence and after tryptic digestion, bovine specific fragments were detected and selected as target compounds for the LC–ESI-MS-MS detection (17). In contrast with other peptide compounds, immunochromatographic purification was not needed because concentrations in blood are fairly high (1 mg/mL).
Beta2-agonists: Beta2-agonists (β2 agonists) including clenbuterol, salbutamol (albuterol), fenoterol, formoterol and salmeterol are bronchodilator drugs. The use of beta2-agonists is prohibited out of competition because of their possible anabolic side effects at high concentrations. Beta2-agonists also are prohibited in competition due to their stimulating effect at higher levels than therapeutically recommended. Because beta2-agonists are used frequently for the treatment of exercise-induced asthma, athletes can obtain a therapeutic use exemption for some beta2-agonists used in metered-dose inhalers.
The extraction and analysis of this group of substances is frequently performed together with other classes of doping substances (Table I). In our laboratory, beta2-agonists are included in the LC–ESI-MS-MS screening method for anabolic steroids and corticosteroids. The sensitivity of LC–MS for this class of substances combined with frequent abuse of inhaled beta2-agonists result in numerous adverse analytical findings (AAF).
In Figure 1, chromatograms obtained after the extraction of a urine sample in which salmeterol was detected are presented together with the chromatograms of a control urine fortified at 0.5 ng/mL. The concentration in the real sample was estimated below 1 ng/mL.
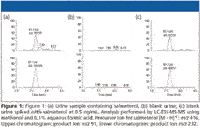
Figure 1
Agents with antiestrogenic activity: This class of substances is divided into aromatase inhibitors (for example, anastrozole, letrozole, exemestane), selective estrogen receptor modulators (SERMs) (for example, raloxifene, tamoxifen, and toremifene), and antiestrogenic substances (for example, clomiphene and cyclofenil).
Most of these substances are used in anticancer therapy. Tamoxifen, for example, is the most widely used drug in breast cancer treatment. In sports, agents with antiestrogenic activity can be used for the treatment of adverse side effects of anabolic steroid abuse like gyneacomastia and reduced testosterone production or to prolong the anabolic activity of administered anabolic steroids by inhibiting the aromatase pathway. Mazzarino and colleagues (6) describe an LC–ESI-MS-MS screening method for 28 doping agents, in which five antiestrogenic drugs were included. Sample preparation consisted of a buffered liquid–liquid extraction at pH 9.2. Detection was performed in positive ionization mode and LODs varied between 5 and 30 ng/mL. Another method describes the detection of 15 antiestrogenic substances (18). After hydrolysis, liquid–liquid extraction at pH 9.2 was used for the sample preparation. LC-ESI-MS-MS analysis was performed in 13 min. The LODs were lower than 10 ng/mL except formestane (300 ng/mL) and aminoglutethimide (100 ng/mL). In this method, only parent drugs were included, whereas according to Mareck and colleagues (19, 20), several antiestrogens metabolites should be screened for. Another paper describes the detection of six antiestrogenic substances using LC–ESI-time-of-flight (TOF) MS after enzymatic hydrolysis and solid-phase extraction (21). For all substances, the LODs were 100 ng/mL.
The metabolism and excretion of 6-OXO, a nutritional supplement sold on the internet as an aromatase inhibitor, was investigated in our laboratory (22,23). This supplement contains androst-4-ene-3,6,17-trione as active ingredient. Androst-4-ene-3,6,17-trione is metabolized to androst-4-ene-6α-ol-3,17-dione and androst-4-ene-6α,17β-diol-3-one. An LC–APCI ion-trap MS method was developed and validated for the quantification of androst-4-ene-3,6,17-trione and its metabolites in urine. Sample clean up consists of a liquid–liquid extraction at pH 9.2 with diethyl ether after enzymatic hydrolysis. Using this method, androst-4-ene-6α-ol-3,17-dione was identified as a major urinary metabolite and androst-4-ene-6α,17β-diol-3-one as a minor metabolite (Figure 2).
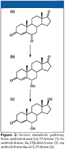
Figure 2
Diuretics and other masking agents: Diuretics are used therapeutically in the treatment of fluid retention. In sports, they do not enhance performance, but could mask the intake of other drugs by diluting the urine (24). Diuretics also are used to lose weight rapidly prior to competition in sports in which weight limits are set, for example, wrestling, judo, and boxing. Diuretics include polar compounds with a wide variety of physico-chemical properties and great differences in their structure. As a consequence of these differences in molecular structure, differences in ionization are observed. Thiazide diuretics represent an important group of diuretics and form abundant deprotonated molecular ions in negative ionization. Canrenone (a diuretic with a steroid skeleton), amiloride and triamterene are exclusively detected in positive ionization. Hence, both polarities are necessary to detect them all. Because these compounds can coelute, scan-to-scan polarity switching is necessary to detect them in a single run. Initially, due to instrument limitations, scan-to-scan polarity switching was not possible and two consecutive runs were necessary (25). By the end of the 1990s, LC–MS instruments allowing reliable and fast polarity switching became available, and positively as well as negatively charged diuretics could be detected in a single chromatographic run. As a consequence, several doping laboratories have developed screening methods using scan-to-scan polarity-switching for the detection of diuretics (26–28) (Figure 3).
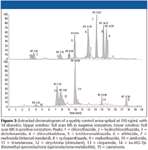
Figure 3
When LC–MS screening for diuretics was introduced in our laboratory in 2001, the method contained 18 diuretics and probenecid. Because in this screening method full-scan MS in both polarities was applied throughout the entire chromatographic run, new compounds easily could be added without modifying the MS-method. Currently, this method is extended to 26 diuretics, 21 beta-blockers, some stimulants, and carboxy-finasteride (28). Carboxy-finasteride is the target compound to detect the abuse of finasteride a 5a-reductase inhibitor (29). This compound exhibits acidic properties and can be extracted easily at pH 5.2 used for the diuretics.
Compounds Prohibited in Competition
Stimulants: The most well known stimulants are amphetamines. In endurance sports requiring intense anaerobic exercise, amphetamines can prolong the tolerance to anaerobic metabolism. Most stimulants are prohibited at any concentration. However, for some ephedrine-type compounds, a threshold is applied (4). This group of substances traditionally is analyzed by GC with nitrogen–phosphorus detection or GC–MS with moderate to good sensitivity. Therefore, routine application of LC–MS for this class of substances is still limited to stimulants that are difficult to detect by GC. Such substances are modafinil, adrafinil, modafinil-acid (a metabolite for adrafinil and modafinil), ritalinic acid (methylphenidate metabolite), mesocarb-hydroxysulphate (mesocarb metabolite), and strychnine (Table I). In our laboratory, these substances are included in a full-scan ion-trap MS screening method for the diuretics. The extracted chromatograms of a urine sample in which modafinil and modafinil-acid were detected are presented in Figure 4. For modafinil, the ions at m/z 296 and m/z 167 are monitored in positive mode while for the acidic metabolite, the ions at m/z 167 and m/z 273 are monitored in negative ionization.
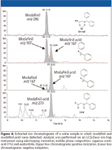
Figure 4
The ion at m/z 296 corresponds to the sodium adduct of modafinil wether the ion at m/z 167 corresponds to a benzyltropylium originating from the neutral loss of the sulfonyl-side chain from modafinil. For modafinil-acid no sodium-adduct was observed in positive ionization, but a benzyltropylium also could be observed (Figure 4). In negative ionization, the [M–H]– could be detected together with a phenyl-benzyl anion. This phenyl-benzyl anion also corresponds to an m/z of 167. The spontaneous fragmentation of modafinil and modafinil-acid into this kathion or anion, stabilized by the aromatic rings, could not be avoided at any temperature of the heated capillary. Another stimulant included in our LC–MS screening method for diuretics is ritalinic. Ritalinic acid is a metabolite of methylphenidate and can be detected much longer in urine than the parent compound (30). Ritalinic acid is screened for by full-scan MS2 monitoring one product ion at m/z 84 (Figure 5). For confirmation purposes, additional diagnostic ions are generated in MS3 (Figure 5). Another application of LC–MS is the quantification of ephedrine and cathine (threshold level 10 and 5 μg/mL, respectively) The sample preparation is limited to filtration of the urine and subsequently injection into the LC–MS system (31).
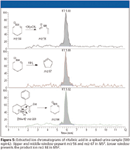
Figure 5
The comprehensive detection of 27 stimulants has been investigated in our laboratory (21,32). The method consisted of a liquid–liquid extraction with diethyl ether at pH 14 and analysis of the extracts with an ion-trap instrument with APCI in positive ionization mode. The total run time was 15 min. All compounds were analyzed in MS2 or MS3. The LODs for all compounds were lower than 25 ng/mL except for chlorphentermine (detection limit: 250 ng/mL). For several substances, LODs of 1 ng/mL were obtained.
Narcotics: In sports, narcotics can be used by athletes to relieve moderate to severe pain, allowing athletes to perform while they actually are injured.
Although several screening methods in other fields have been published, routine LC–MS screening of narcotics for antidoping purposes has been described only recently by LC–ESI-TOFMS (21). In this approach, narcotics were detected after enzymatic hydrolysis and solid-phase clean up (Table I).
The detection of narcotics by LC–ESI-MS-MS was investigated in our laboratory (33). Chromatography was performed on a C18 column, starting at a low percentage of organic modifier to improve the retention of morphine and hydromorphone. High percentages of organic modifier are necessary for the elution of hydrophobic compounds including buprenorphine and methadone. As internal standard nalorphine was used. Two extraction solvents were evaluated, methylene chloride (CH2Cl2) and diethyl ether. With diethyl ether LODs ranged between 0.5 and 20 ng/mL and with methylene chloride LODs ranged between 0.5 and 10 ng/mL.
Morphine is the only narcotic with a threshold level of 1 μg/mL. A LC–ESI-MS-MS method has been described for its quantification by direct injection of the urine into the LC–MS system after filtration (31). In this way both morphine and morphine-glucuronides can be detected without the need for hydrolysis and extraction in GC–MS analysis.
Glucocorticosteroids: Corticosteroids are very powerful anti-inflammatory agents used for the treatment of inflammatory diseases. Before the introduction of LC–MS, their detection was performed by GC–MS. Selectivity was sufficient, but a time-consuming derivatisation step was necessary due to the low volatility of most corticosteroids. In the second half of the 1990s, LC–MS research revealed that corticosteroids were ideal compounds for LC–MS. They can be detected with both ESI and APCI, in both polarities. In positive ionization mode corticosteroids can be detected by protonated molecular ions. Because corticosteroids have no protons with a gas-phase acidic behavior (34), no [M–H]– can be observed in negative mode. Instead, they form very abundant adducts with acetic or formic acid, which are used frequently as mobile phase additives (Figure 6). This fact has been used to distinguish between endogenous corticosteroids and endogenous anabolic steroids, which do not form such adducts in negative mode (14). Because corticosteroids and anabolic steroids only differ in the presence of the C21 and C22, it can be supposed that the keto at C21 and the hydroxyfunctions at C20 and C22 are involved in the adduct formation in negative ionization (Figure 6).
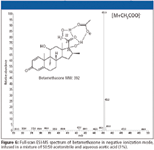
Figure 6
In our initial corticosteroid LC–MS-MS screening method, negative ionization was preferred. LODs of this method ranged between 0.5 and 5 ng/mL. Currently, glucocorticosteroids are included in the LC–MS-MS screening method for anabolic steroids (nonpublished results).
A corticosteroid frequently detected during routine analysis is 16α-hydroxyprednisolone. 16α-hydroxyprednisolone is the target metabolite for budesonide (35). Budesonide is a corticosteroid available in a metered dose inhaler and is prescribed frequently for the treatment of exercise-induced asthma.
Conclusions and Future Challenges
Several compounds described here were analyzed using full-scan ion-trap MS. Although full-scan MS spectra are useful for the identification of the analytes based upon their protonated or deprotonated molecular ions, they do not provide sufficient structural information for definite identification. Hence, tandem MS is used to obtain three diagnostic ions (36). APCI and ESI are widely accepted ionization interfaces in doping analysis. Despite the introduction of atmospheric pressure photo ionization (APPI), atmospheric pressure laser ionization (APLI) or heated electrospray ionization (HESI), these ionization techniques have not yet found routine applications in doping analysis.
Currently, routine LC–MS applications are available for almost all classes of doping substances. Improvements in MS-technology with respect to scan speed, sensitivity as well as polarity switching allow for the combined detection of different classes of doping agents (Table I). Because of the expanded use of LC–MS, the number of manufacturers producing LC–MS systems has increased and consequently, the purchase costs are decreasing. Hence, LC–MS instruments, formerly too expensive, are becoming affordable. New techniques include particularly high-resolution LC–MS techniques. These high-resolution technologies are very promising in structure elucidation of unknown compounds due to accurate mass measurements. One of the high-resolution techniques is time-of-flight (TOF) MS, which allows for the detection of several hundreds of substances with high specificity and sensitivity (21,37). Because of the full mass range, new compounds can be added to the developed method easily. TOF-MS analyzers also provide a promising tool for peptide indetification due to their extended mass range.
Acknowledgments
Postdoctoral grants by the Flemish Ministry of Culture, Youth, Sports and Brussels (PVE and KD) and the Spanish Ministerio de Educacion y Ciencia (OJP) are gratefully acknowledged. Technical assistance by Kris Roels was greatly appreciated.
K. Deventer*, O.J. Pozo*† , P. Van Eenoo*, and F.T. Delbeke*
*DoCoLab, UGent, Department of clinical chemistry, microbiology and immunology
Technologiepark 30, B-9052 Zwijnaarde, Belgium
† Research Institute for pesticides and water, University Jaume I, E-12071, Castellón, Spain
Please direct correspondence to Koen.Deventer@UGent.be
References
(1) F.T. Delbeke. Biology of Sport 17, 81–86 (2000).
(2) R. Ventura, D. Fraisse, M. Becchi, O. Paisse, and J. Segura, J. Chromatogr. Biomed. Appl. 562, 723–736 (1991).
(3) L. Politi, A. Groppi, and A. Polettini, J. Anal. Toxicol. 29, 1–14 (2005).
(4) WADA, The World Anti-Doping Code,The 2007 Prohibited List, International Standard, 2007.
(5) WADA, 2006 Adverse analytical findings reported by accredited laboratories, 2006.
(6) M. Mazzarino and F. Botre, Rapid Commun. Mass Spectrom. 20, 3465–3476 (2006).
(7) K. Deventer, P. Van Eenoo, and F.T. Delbeke, Biomed. Chromatogr. 20, 429–433 (2006).
(8) M. Thevis, G. Fussholler, H. Geyer, G. Rodchenkov, U. Mareck, G. Sigmund, A. Koch, A. Thomas, and W. Schanzer, Chromatographia 64, 441–446 (2006).
(9) O.J. Pozo, P. Van Eenoo, K. Deventer, and F.T. Delbeke, J. Mass Spectrom. 42, 497–516 (2007).
(10) O.J. Pozo, P. Van Eenoo, K. Deventer, and F.T. Delbeke, Analytical and Bioanalytical Chemistry 389, 1209–1224 (2007).
(11) M.H. Sekera, B.D. Ahrens, Y.C. Chang, B. Starcevic, C. Georgakopoulos, and D.H. Catlin, Rapid Commun. Mass Spectrom. 19, 781–784 (2005).
(12) M. Thevis, U. Bommerich, G. Opfermann, and W. Schanzer, J. Mass Spectrom. 40, 494–502 (2005).
(13) W. Schanzer, H. Geyer, G. Fussholler, N. Halatcheva, M. Kohler, M.K. Parr, S. Guddat, A. Thomas, and M. Thevis, Rapid Commun. Mass Spectrom. 20, 2252–2258 (2006).
(14) O.J. Pozo, K. Deventer, P. Van Eenoo, and F.T. Delbeke, Rapid Commun. Mass Spectrom. 21, 2785–2796 (2007).
(15) M. Thevis, A. Thomas, P. Delahaut, A. Bosseloir, and W. Schanzer, Anal. Chem. 77, 3579–3585 (2005).
(16) M. Thevis, M. Bredehoft, H. Geyer, M. Kamber, P. Delahaut, and W. Schanzer, Rapid Commun. Mass Spectrom. 20, 3551–3556 (2006).
(17) M. Thevis, R.R. O. Loo, J.A. Loo, and W. Schanzer, Anal. Chem. 75, 3287–3293 (2003).
(18) M.J. Kang, Y.H. Hwang, W. Lee, and D.H. Kim, Rapid Commun. Mass Spectrom. 21, 252–264 (2007).
(19) U. Mareck, H. Geyer, S. Guddat, N. Haenelt, A. Koch, M. Kohler, G. Opfermann, M. Thevis, and W. Schanzer, Rapid Commun. Mass Spectrom. 20, 1954–1962 (2006).
(20) U. Mareck, G. Sigmund, G. Opfermann, H. Geyer, M. Thevis, and W. Schanzer, Rapid Commun. Mass Spectrom. 19, 3689–3693 (2005).
(21) M. Kolmonen, A. Leinonen, A. Pelander, and I. Ojanpera, Anal. Chim. Acta 585, 94–102 (2007).
(22) W. Van Thuyne, P. Van Eenoo, P. Mikulcikova, K. Deventer, and F.T. Delbeke, Biomed. Chromatogr. 19, 689–695 (2005).
(23) K. Deventer, P. Van Eenoo, P. Mikulcikova, W. Van Thuyne, and F.T. Delbeke, J. Chromatogr., B, 828, 21–26 (2005).
(24) F. T. Delbeke and M. Debackere, Biopharm. Drug Disposition 9, 137–145 (1988).
(25) D. Thieme, J. Grosse, R. Lang, R.K. Mueller, and A. Wahl, J. Chromatogr., B 757, 49–57 (2001).
(26) C. Goebel, G.J. Trout, and R. Kazlauskas, Anal. Chim. Acta 502, 65–74 (2004).
(27) Y. Qin, X.B. Wang, C. Wang, M. Zhao, M.T. Wu, Y.X. Xu, and S.Q. Peng, J. Chromatogr., B 794, 193–203 (2003).
(28) K. Deventer, P. Van Eenoo, and F.T. Delbeke, Rapid Commun. Mass Spectrom. 19, 90–98 (2005).
(29) M. Thevis, H. Geyer, U. Mareck, U. Flenker, and W. Schanzer, Therapeutic Drug Monitoring 29, 236–247 (2007).
(30) A. Solans, M. Carnicero, R. Delatorre, and J. Segura, J. Chromatogr., B 658, 380–384 (1994).
(31) M.-H. Spyridaki, P. Kiousi, A. Vonaparti, P. Valavani, V. Zonaras, M. Zahariou, E. Sianos, G. Tsoupras, and C. Georgakopoulos. Anal. Chim. Acta 573, 242–249 (2006).
(32) K. Deventer, P. Van Eenoo, and F.T. Delbeke, Rapid Commun. Mass Spectrom. 20, 877–882 (2006).
(33) K. Deventer, O.J. Pozo, P. Van Eenoo, and F.T. Delbeke. Rapid Commun. Mass Spectrom. 21, 3015–3023 (2007).
(34) A.P. Bruins, J. Chromatogr., A 794, 345–357 (1998).
(35) K. Deventer, P. Mikulcikova, H. Van Hoecke, P. Van Eenoo, and F.T. Delbeke, J. Pharm. Biomed. Anal. 42, 474–479 (2006).
(36) WADA, Technical Document, TD2003IDCR, 2007.
(37) M.E. Touber, M.C. van Engelen, C. Georgakopoulus, J.A. van Rhijn, and M.W.F. Nielen, Anal. Chim. Acta 586, 137–146 (2007).

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).













