Data Integrity in the GxP Chromatography Laboratory, Part II: Setting up a Chromatograph and Acquiring Data
LCGC North America
Part II of this series on practical perspectives on data integrity focuses on instrument setup, system suitability test samples, and data acquisition.
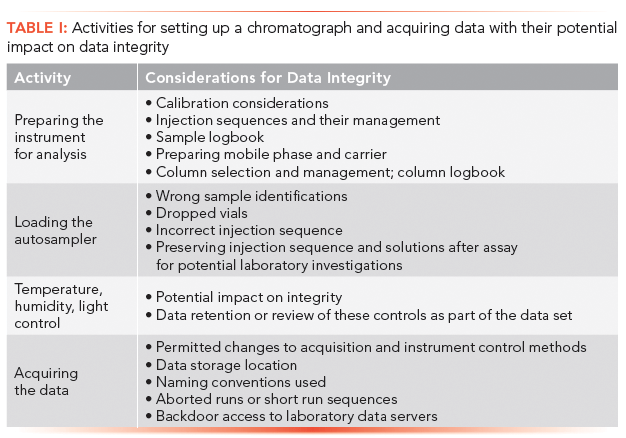
Correct, complete, and accurate data acquisition is a key component of data integrity. Here we look at how this component should be applied to a chromatograph and a chromatography data system (CDS). In this article, we discuss how to ensure the correct setup of an instrument, run system suitability test samples, and acquire data.
In the first article in this series (1), we covered the sampling and sample preparation phases of analysis and the vials ready for analysis. In this article, we look at what should be done to ensure the correct set up of an instrument, run system suitability test samples, and acquire data. The assumptions we have made are that the chromatograph is qualified and the chromatography data system (CDS) is configured for protecting electronic records, validated, and ideally uses electronic signatures. There should not be any shared user identities so actions in the CDS can be attributed to an individual. For further reading there is an earlier series about the ideal CDS for a regulated laboratory (2–5) and a recent book on the validation of CDS (6). We will not be discussing the instrument logbook because that was the subject of a recent publication (7).
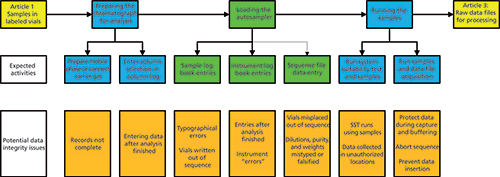
Figure 1: Setting up the chromatograph and acquiring data.
Figure 1 shows what is discussed in this article, and that is expanded in Table I.
Managing Factors, Weights, and Other Assay Values in Calculations
Chromatography assays often require the input of additional data values that are combined with the chromatographic value to generate data values that are compared against specifications. For example, the estimated potency, based on the chromatography injection, is combined with the sample weight and moisture results to determine the anhydrous potency, which is the potency value that determines the fitness of the product for release (specification test). Some biologics may have potency or conversion factors that are applied. Whatever the specifics, chromatography methods require the input of other (external) values to generate results that will be compared to specifications.
These additional values are critical, and directly impact the accuracy of the calculated result; any error in a value will create a corresponding error in the calculated value in every occurrence. Therefore, they must be right. So, how do we get them? Start in a laboratory information management system (LIMS) (or the analytical method) to look up the correct value, then manually enter the value into the electronic method. Some laboratories have developed automated tools to transfer these values from an electronic laboratory notebook (ELN) or laboratory execution system (LES). Automated transfer of these external values can eliminate human transcription errors, but it poses integrity risks of its own if not properly configured and managed:
- Data values manually entered into an ELN or LES, then electronically transferred into a chromatography method, have the same human error risks as data values entered directly into the chromatography system, unless the ELN or LES has some validity checks or other risk-reduction actions embedded in them at the time of data entry.
- When interface files are placed in a target directory to be later collected and processed into the chromatography system, the target directory provides a convenient place for someone to insert an altered data value into the chromatography calculations. This type of manipulation can go undetected, especially if done infrequently. There may be no audit trail for actions in the target directory, and when an audit trail is available, it may be seldom reviewed. A manipulated data set might have a sample weight slightly different from the value in the ELN or LES system-a value that will "improve" the calculated value so it meets the specification. Analysts trust the imported data, because it came in through a validated interface. Only by comparing the chromatography data with the original value can this type of manipulation be discovered.
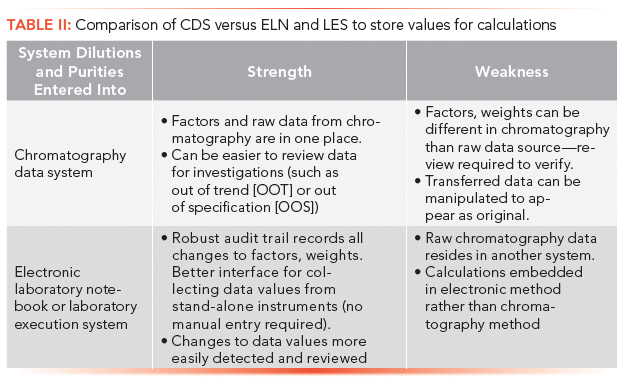
An alternate approach to calculations is available when the laboratory uses an ELN or LES system. Rather than entering or transferring these additional data values into the chromatography system for calculations, do the opposite: Transfer the basic chromatographic data into the ELN or LES, and perform the post-assay calculations there. Which approach is better? It probably depends on the chromatography system and ELN or LES system in question. In general, data integrity would lead you toward calculations in a location where there is a more robust audit trail, where changes in values are most easily detected, and where recalculations are more restrictive and controlled. Some ELN systems do not-or can be poorly configured to not-provide audit trail records on every action. In contrast, many LES systems create audit trails for every action where any value is overwritten. Chromatography systems vary in their audit trails around calculation and factor changes. The final decision should provide the most robust, secured, and detectable environment for entry and management of external factors, weights, and assay results, as shown in Table II.
Obfuscation as a Manipulation Tool
One technique for the willful data manipulator is obfuscation-piling on layers of data to make the truth more difficult to know, to the point that the reviewer finally gives up and accepts the value as genuine. With a chromatography system, obfuscation is easily accomplished by sending multiple data files to a single chromatography run, then perhaps a manual override of a value, followed by another round of data transfer. The resulting audit trail is a tangled weave of changes and timestamps that could be understood-but only with hours of tedious digging. Given other constraints, and the desire to go home at some point, reviewers are tempted to accept the value on their screen and move on. And the manipulator wins. Systems with clear audit trails, formatted for easy review, help to streamline reviews and make it more difficult to use obfuscation. Unfortunately, some systems combine audit trails, adding to the review woes. Quality reviewers should look for test patterns where numerous changes are made, and dig into the data to verify the scientific validity of changes, to stem the use of obfuscation.
System Suitability Test Management
Chromatographic methods have some series of injections that verify the suitability of the instrument before reference standard and sample injections are made. Failure of the suitability test is justification for invalidation of all subsequent standard and sample injections in the test run; therefore, data reviewers must look cautiously at the suitability process, especially when the test run is to be declared invalid along with test results, such as the situation where the system suitability sample, standard, and samples were placed in an autosampler and assayed overnight without human intervention. There are two possible issues:
1. the system suitability samples failed acceptance criteria;
2. the test results failed, and now the analyst is seeking to invalidate the run by manipulating the suitability results.
When test results have been generated, a wise reviewer should look at the potential data values for potential failures as part of the system suitability review.
The potential for system suitability abuse begs a question: Will failures result in investigation and corrective measures? The answer to this question should be included in your procedure on chromatography. In addition, your procedure should also describe the conditions when it is acceptable to perform other types of injections not specified in the method (unplanned), such as injections to verify correct system response before starting system suitability, drift injections, or others. Your chromatography procedure should answer the following questions:
- When are extra (unplanned) injections acceptable?
- If they fail to meet expectations, will they result in repeats or investigations?
- How many repeats are acceptable? When do they stop?
- How will the unplanned injections be reviewed to determine scientific validity?
In a regulated environment, where equipment is qualified and software and analytical methods are validated and users are trained, every injection must be part of the good manufacturing practice (GMP) record.
Incomplete (Aborted) Runs
While collecting data, it may be necessary to abort a run because of any number of issues that impact the test run. But there is also a dark side: Like system suitability, aborted runs can be used as a means of stopping the chromatography system from generating undesired (out of specification [OOS]) test results. Rather than managing the test result, the run is simply aborted, preventing the creation of a result value.
For this reason, it is necessary to manage aborted runs as unplanned events, and review the system for the number of aborted runs, along with the group, person, method, instrument, and abort reason comment associated with the aborted run. The review should look for trends in aborted runs. In addition, it is important to include aborted runs with the subsequent test record, so the aborted data are reviewed along with the acceptable data before test result release. The management of aborted runs should be included in your chromatography procedure.
Other Unplanned Injections
There are circumstances where it may be justified to perform an additional injection of a solution, such as a standard or suitability solutions. As for other solutions described above, it is important to specify the circumstances that warrant these injections, the number of injections (re-injections) that is acceptable, how the results will be used, and confirming that the injection results will be reviewed with all associated test records, to ensure retention of the complete testing record.
Data Output
After the assay has met suitability criteria and injections have been collected, the raw injection results must be stored for processing and assay calculations. One issue that has found its way into some laboratories is diversion of test data into "alternate" directories. This step is more difficult to carry out with relational database systems, but can be readily done with file-based chromatography systems. For example, test runs with failing injections are placed into a folder named "Test" to make them appear as non-production test results (11). This type of behavior can be detected by creating a report to inventory test runs referenced in reported test results, looking for missing runs in the sequence (each run is given a unique identification by the system). A second detection report can look for the presence of folders or injections with suspicious names like "Test," "Practice," or other terms that merit review.
To ensure data integrity, laboratory personnel must be trained to submit every injection for data review. When data are rejected for use, a reason for that rejection must be documented to defend the data exclusion from the reported values.
Is Management the Problem?
Management sets the tone for the laboratory and enforces the expectations. They steer the culture of the firm. Throughout the process of instrument setup and data collection, management must set the expectation to follow the chromatography procedure and fully report all values generated-whether used for calculations or not. Management is responsible for ensuring that the system is capable of securing data during the testing process, and is configured to capture actions in audit trails that assist the data reviewer to understand the actual actions that occurred. It is especially important for management to expect unplanned injections to be minimized, and not used as a substitute for investigating a substandard instrument or method to understand failures and prevent their recurrence.
Summary
In the second part of this six-part series, we moved from the manual portion of the analysis to the start of computerization and looked at the data integrity issues associated with preparing the chromatograph for analysis and running the samples.
Part III of this series will focus on what should be done to control integration and interpretation of the chromatographic files.
References
(1) M.E. Newton and R.D. McDowall, LCGC North Am. 36(1), 46–51 (2018).
(2) R.D. McDowall and C. Burgess, LCGC North Am. 33(8), 554–557 (2015).
(3) R.D. McDowall and C. Burgess, LCGC North Am. 33(10), 782–785 (2015).
(4) R.D. McDowall and C. Burgess, LCGC North Am. 33(12), 914–917 (2015).
(5) R.D. McDowall and C. Burgess, LCGC North Am. 34(2), 144–149 (2016).
(6) R.D. McDowall, Validation of Chromatography Data Systems, 2nd Edition (Royal Society of Chemistry, Cambridge, UK, 2017).
(7) R.D. McDowall, Spectroscopy 32(12), 8–12, 28 (2017).
(8) Semler Research Center Private Limited, FDA Untitled Letter, April 2016 https://www.fda.gov/Drugs/DrugSafety/ucm495778.htm.
(9) USV Limited, FDA Warning Letter, February 2014 http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm386678.htm.
(10) RPG Life Sciences Warning Letter, May 2013 http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm355294.htm.
(11) Hospira SpA Warning Letter, March 2015 http://www.fda.gov/ICECI/Enforcement-Actions/WarningLetters/ucm440966.htm.
Mark E. Newton is the principal at Heartland QA in Lebanon, Indiana. Direct correspondence to: mark@heartlandQA.com. R.D. McDowall is the Director of RD McDowall Limited in the UK. Direct correspondence to: rdmcdowall@btconnect.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).











