Is Cyclohexane a Suitable HPLC Solvent? It Can Become Solid Under Pressure
LCGC Europe
If cyclohexane is mixed with even low amounts of other solvents, the melting pressure increases remarkably.
Pure compounds appear in various phases. Depending on the temperature and pressure, they can be a solid, a liquid, a gas or a supercritical fluid. Typical behaviour of the different phases is shown schematically in Figure 1. Many compounds have a triple point pressure well below 1 bar whereas the critical point is in the order of magnitude of 100 bar. Nevertheless, it is obvious that such a compound becomes solid if the pressure is high enough, depending on the temperature. The line that separates the solid from the liquid state is the so-called melting pressure curve. Usually we have no experience with it because we live in a 1?bar world; in contrast, we learnt about the boiling point curve when doing experiments with vacuum distillation during our laboratory education. (It is worth noting that some compounds, such as water, show anomalous behaviour and have a melting pressure curve with negative slope. This is why ice melts under pressure.)
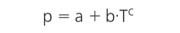
HPLC separations run under pressures of 30–300 bar and even higher. Nowadays, pumps and equipment are available that expand the usable range to 1000 bar allowing faster separations or the use of longer columns (i.e., higher plate numbers).1,2 High pressure is a very interesting option but the properties of the mobile and stationary phase, as well as of the column material, can be different from the well-known ones.3 Some solvents have remarkably low melting pressures at 20 °C:3
- p-xylene, 191 bar
- cyclohexane, 251 bar
- 4-chlorotoluene, 451 bar
- bromoform, 487 bar
- benzene, 518 bar.4
These are compounds with melting points above 5 °C at 1?bar (i.e., they freeze when stored in a refrigerator). We investigated the behaviour of cyclohexane because it is a solvent not uncommon in the laboratory. Although it is probably only very rarely used for analytical HPLC it is the preferred solvent for some preparative liquid chromatographic separations because of technical requirements (for example, a crystallization step in cyclohexane just after the separation or an interesting selectivity for certain applications).5
The Melting Pressure of Cyclohexane
A paper from 1916 implicitly notes that pure cyclohexane becomes solid between 200 and 400 bar.6 The authors investigated the compressibilities of various organic solvents at 20 °C. For the relationship between pressure and melting temperature an equation was proposed in 1929 by Simon and Glatzel, now called the Simon equation.7 It is noted here in an adequately simplified form:
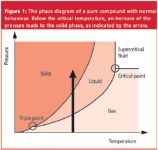
Figure 1
with p = pressure in MPa (1 MPa = 10 bar) and T = temperature in Kelvin.
Carefully investigated parameters a, b and c for some n-alkanes and cyclohexane can be found in a recent paper. For cyclohexane a = -1090, b = 39.035 and c = 0.590.8
With these parameters we achieve melting pressures of 236 bar at 20 °C, 348 bar at 25 °C, 459 bar at 30 °C, or 1003 bar at 55 °C. The melting pressure curve between 10 and 55 °C is shown in Figure 2. Cyclohexane has a melting point of 6.6 °C at 1 bar. (There is a discrepancy between the melting pressure values at 20 °C noted here and above, namely 236 bar versus 251 bar. It comes from the fact that the original Simon equation is not trivial to solve9 and that the necessary experiments for the determination of the parameters are demanding.)10

Figure 2
If cyclohexane is mixed with even low amounts of other solvents, the melting pressure increases remarkably. To mention but two examples: With a mole fraction of 0.10 benzene in cyclohexane the melting pressure at 20°C is 713 bar;4 or with a mole fraction of 0.04 tridecane at the same temperature it is 416 bar.8
The critical point of cyclohexane is at 281 °C and 41 bar.
Experimental
Cyclohexane in an HPLC pump: Around 1980, Henri Colin was wondering why the sapphire piston of his Waters M 6000 pump was breaking repeatedly.5 (Piston breakings were not uncommon in those days because the pumps were operating with conventional electric motors, controlled by the current. If a pump was run under 'constant flow' and the selected flow-rate could not be achieved, the motor was turning to full power with high torque.) Finally, Colin found out that it was the eluent, cyclohexane, which provoked the problem because it solidified under pressure. It is not trivial to detect this reason because the eluent becomes liquid immediately when the pressure decreases. Solid cyclohexane is never visible during such an experiment!
We investigated the behaviour of a Jasco PU-980 HPLC pump (Jasco, Tokyo, Japan) with pure cyclohexane (LiChrosolv for Chromatography, Merck, Darmstadt, Germany). It is a dual-piston pump with a displacement of 37.3 µL per stroke, which is driven by a stepper motor. Such motors operate with lower torque. The pressure can be monitored with a signal of 20 mV per 100 bar. An old HPLC column was connected to the pump to yield the necessary backpressure. The study was performed in a laboratory with a constant temperature of 23 °C. The Simon equation predicts a melting pressure of 303 bar under these conditions.
The proper operation of the pump was checked with flow-rate determinations by measuring flask and stop-watch. The measured flow-rates were slightly too low, for example, 0.49 mL/min instead of 0.50 mL/min but since the instrument was an old pump without recent service we accepted them as adequate. However, at a set flow of 0.7 mL/min (displayed pressure approximately 350 bar) the experimental value dropped to 0.47 mL/min. The motor stopped working in frequent but irregular intervals for short periods of time. At both nominal flows of 0.8 mL/min and 0.9 mL/min the experimental data were identical, namely a displayed pressure of 360 bar and a flow-rate of 0.65 mL/min.
This means that the pumping function was grossly disturbed, a fact that was also obvious by acoustical and visual inspection (the pump housing had been removed). The pumping behaviour became accidental with long intervals of piston blocking when the flow-rate was set to 1.0 mL/min. The experimental flow-rate dropped to 0.60 mL/min but this value is a random result. After increasing the set value to 1.1 mL/min the pressure display indicated 390 bar and the pump stopped working after a short period of time. The pistons did not break and the pump was not damaged; at lower flow-rates it worked properly again.
Figure 3 shows the pressure signal during a flow-rate step gradient. The flow was increased by 0.1 mL/min every two minutes from 0.1 mL/min to 1 mL/min. It can clearly be seen that the pump operation was undisturbed up to 0.6 mL/min but became erroneous once the pressure was clearly higher than 300 bar. During this experiment the instrument stopped working at a set flow-rate of 1.0 mL/min, again without any damage.
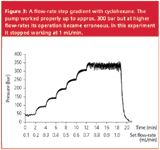
Figure 3
Conclusions
It seems that it is not possible to provoke a piston breakage with today's HPLC pumps, equipped with stepper motors that excite lower torque. However, the proper operation is not possible once the combination of flow-rate and column permeability gives a pressure that is higher than the melting pressure of the eluent. If cyclohexane should be tested or used as an HPLC mobile phase it is best to set the upper pressure limit below the melting pressure as predicted by the Simon equation at the temperature of operation. By increasing the working temperature the problem can easily be evaded since the melting pressure curve is steep. The phenomenon of solidifying eluents could become more than a very rare event with the expected increase of the pressure range in HPLC in the future.11 In addition, the technology of column packing requires high pressures.
Acknowledgement
We thank Mr Sergio Rezzonico of EMPA St. Gallen for technical support with the pump experiment and the data acquisition.
Bruno E. Lendi was a product and business manager for analytical instruments. The company founded by him and his partner is OmniLab Ltd in Switzerland, supporting chromatography, spectroscopy and labware. Veronika R. Meyer, PhD, is a specialist in HPLC and measurement uncertainty. She works as a research scientist at EMPA St. Gallen, Switzerland, and teaches at the University of Bern.
References
1. I. Halász and G. Görlitz, Angew. Chem., 94, 50–62 (1982).
2. M. Swartz and B. Murphy, LabPlus Int., 18(3), 6–9 (2004).
3. M. Martin and G. Guiochon, J. Chromatogr. A, 1090, 16–38 (2005).
4. K. Nagaoka and T. Makita, Int. J. Thermophysics, 8, 415–424 (1987).
5. H. Colin, Novasep, F-Pompey, personal communication, Jan. (2006).
6. T.W. Richards and J.W. Shipley, J. Am. Chem. Soc., 38, 989–999 (1916).
7. F. Simon and G. Glatzel, Z. Anorg. Allgem. Chem., 178, 309–316 (1929).
8. U. Domańska and P. Morawski, Fluid Phase Equilibria, 218, 57–68 (2004).
9. S.E. Babb, Jr., Revs. Modern Physics, 35, 400–413 (1963).
10. B. Baranowski, Polish J. Chem., 52, 1789–1798 (1978).
11. J.R. Mazzeo et al., Anal. Chem., 77, 460A–467A (2005).

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.














