Article
LCGC North America
LCGC North America
Csaba Horváth and the Development of the First Modern High Performance Liquid Chromatograph
Author(s):
Columnist Leslie Ettre looks back at the life and contributions of Csaba Horvath, one of the giants in the field of chromatography.
The development of modern high performance liquid chromatography (HPLC) is connected closely with the activities of Csaba Horváth, professor at Yale University (New Haven, Connecticut), who died unexpectedly one year ago. His primary contribution was to transform classical liquid column chromatography — which even 60 years after its invention by M.S. Tswett still was being performed essentially in the same way as first described by its inventor — into a modern instrumental separation technique that became the most widely used laboratory method in chemistry and biochemistry within about two decades.

Leslie S. Ettre
Csaba carried out his fundamental work from 1964 to 1966. We often discussed the desirability that, similar to other early contributors, he should summarize his personal recollections on these exciting years. In the past, he wrote two short autobiographical treatments (1,2), but in these, he touched only briefly on the development of the instrument. We agreed that the 40-year anniversary of his work would be an excellent opportunity to finally provide a report on it, and he planned to add a contribution to this very column. His untimely, sudden death prevented him from doing this.
Since his arrival in the United States 40 years ago, I had the good fortune to maintain close contact with Csaba, both socially and professionally. Although I have not been involved directly in his work, we discussed it continuously and thus, I was aware of his ideas, trials and tribulations, and results. Thus, I decided to use the one-year anniversary of his death to fill in the gap in the history of HPLC development, and summarize the circumstances that led to the development of HPLC as a modern analytical technique and the construction of the first modern, high-pressure liquid chromatograph.
During the past year, numerous articles and editorials have eulogized Csaba, discussing in detail his activities in a wide range of fields and here, I only want to cite a few (3–8). It is neither my intention to simply add one more to this list nor to try to compete with them. I will concentrate only on instrument development and place Csaba's activities in the proper historical context.
The Status of Chromatography Around 1960
In the 1950s, gas chromatography (GC) underwent a meteoric development. Over a dozen companies marketed simple-to-use instruments, permitting the use of GC as a routine analytical tool. Within a few years its theory was fully developed allowing prediction of further improvements. In fact, in 1961,
Chemical & Engineering News
could express the opinion that "if ever analytical chemistry is dominated by a single technique, gas chromatography is well on its way to becoming this technique" (9).
The development of GC was a logical consequence of the development in LC. The idea of using a gas instead of a liquid as the mobile phase was first suggested by Martin and Synge in 1941 (10), and after attempts by a few researchers, the advantages of the new technique were demonstrated by James and Martin in 1952 (11). By then, LC was already 50 years old. For 40 years, not much was changed in the way separation was performed, using small glass tubes packed with an adsorbent and simply using gravity to direct the flow of the mobile phase (the eluent). Then, in 1941, Martin and Synge described liquid–liquid partition chromatography (the work for which they received the 1952 Nobel Prize in Chemistry), and in their paper, they predicted clearly that to further improve the columns' separation power, very small particles and high pressures would be needed (10). However, they also stated that due to technical problems, these two goals had to be abandoned, at least temporarily. Then, within a couple of years, Martin and colleagues described paper chromatography (12). This new version of liquid partition chromatography provided such a breakthrough in separation that nobody bothered to improve liquid column chromatography further.
The situation changed with the advent of GC. By the beginning of the 1960s, researchers well versed in the theory of GC and in its technical details started to apply their knowledge to the improvement of liquid column chromatography. By generalizing the theory of separation developed for GC, it became apparent that the limitation in LC was the diffusion in the liquid mobile phase that was about three orders of magnitude slower than in a gas. Thus, to obtain speed and efficiency in LC comparable to GC, this handicap had to be overcome by other means. This possibility was clearly formulated in 1963 by J.C. Giddings (13) who, based upon the general theory of chromatography, pointed out the need for very small particles and high pressure along the column. This was, of course, the same conclusion derived empirically 20 years earlier by Martin and Synge. However, just as they stated at that time, the proper materials and systems still were not available.
The Beginning of Modern LC
Since 1952, S.R. ("Sandy") Lipsky (1924–1986) had been associated with Yale University's Medical School, where he pioneered in the use of GC for the analysis of long-chain saturated and unsaturated fatty acids associated with triglycerides in humans, and had a role in the development of the electron-capture detector. Sandy had an extraordinary sense for picking up new ideas that had the potential to succeed and change the course of science. Thus, in the months after the paper by Giddings, he started to consider the feasibility of improving LC. As he mentioned in his autobiography (14), he was reminded constantly by his colleagues in the medical school of all the biological substances that could not be separated by GC. He also was selected by NASA as one of the principal investigators for the detection of organic substances on the rocks that eventually would be brought back from the Moon. This gave him an opportunity to assemble a team, and it could be foreseen that they would have plenty of time for other investigations before the rocks arrived. Among other things, he asked me whether I knew anyone interested in such activities. I recommended Csaba Horváth.
I had known Csaba well for over a decade. He was my wife's classmate at the Technical University, in Budapest, Hungary. After graduating as a chemical engineer in 1952, he did research at the university. Then, toward the end of 1956, he left Hungary, settling in Germany, where he became associated with one of the large chemical companies as a process engineer. In 1961, he decided to go back to university and earn a Ph.D. degree. He joined at the University of Frankfurt, Frankfurt, Germany, the group of István Halász, who at that time was involved in GC research. As his thesis work, investigations into the practicality of the suggestion of M.J.E. Golay were selected. Golay, the inventor of the open-tubular (capillary) columns, mentioned in his lecture at the Third International Gas Chromatography Symposium held in Edinburgh, U.K., in June 1960, that capillary column performance could be further improved by increasing the inside coated surface of the tubing without increasing its diameter and further coating this increased surface with a very thin stationary phase film (15). Csaba was very experienced in surface chemistry, and this knowledge was very handy in being able to develop a way to coat a porous layer onto the inside surface of the capillary tubes. The result of his research was the support-coated, open-tubular column which, for almost a decade, enjoyed wide popularity (16). In addition, he used the same technology to coat glass beads with a thin layer of porous support or adsorbent and use these particles in packed columns. He named this material pellicular particles (17–19). Further investigations revealed that their use has no particular advantages in GC; however, as discussed in the following, they later became very suitable for LC.
After receiving his doctorate in the spring of 1963, Csaba decided not to go back to the industry (as originally planned) but to stay in university research, and accepted a postdoctoral position at the Physics Research Laboratory of Harvard Medical School, Cambridge, Massachusetts. He became involved in studying the products formed in the irradiation of cholesterol and was horrified when he realized how primitive the LC techniques used in this analysis were. As an experienced gas chromatographer, he was thinking logically about the desirability of having a liquid chromatograph with full control of the operational parameters. Csaba even submitted a proposal to his superiors to develop such an instrument, but he was turned down. No one at Harvard Medical School had any interest in such a project.
In these months, I had frequent contacts with Csaba. Perkin-Elmer (Norwalk, Connecticut), where I was the head of the GC Applications Laboratory, was busy adapting Csaba's methodology to produce support-coated, open-tubular columns. I was well aware of his frustration with Harvard's disinterest in improving LC and thus, when I learned from Sandy Lipsky about his plans, I suggested that he invite Csaba to join him at Yale. As a result, Csaba and his wife moved in the fall of 1964 to New Haven, where he became a research associate at Yale's Medical School.
The next year was most remarkable in Csaba's professional life. His first task was to build a system, an instrument for LC. Knowing the existing literature, he immediately realized that high pressures and very small stationary phase particles would be needed. Within a couple of months, he assembled a high-pressure system for LC, but had problems finding the necessary column packing. At that time, particle technology was not advanced enough, and uniformly sieved, very small diameter particles that would be stable at the high pressures simply were not available. Here, his previous thesis work suddenly became very useful. The pellicular particles with a thin, porous adsorbent layer fulfilled the criteria set over 20 years earlier by Martin and Synge. The very thin porous-layer coating on the surface of the glass beads provided the short diffusion paths needed to overcome the slowness of diffusion. Csaba also was able to pack this material into long (1–2 m), narrow-bore (about 1 mm i.d.) columns and successfully used these in the reversed-phase mode. Figure 1 shows one of his early chromatograms obtained in the winter of 1964–1965. Here, a mixture of free fatty acids was analyzed, the same type of sample the analysis of which Sandy Lipsky pioneered 10 years earlier by GC, and the column packing consisted of pellicular particles coated with a thin layer of graphitized carbon black.
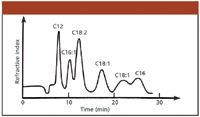
Figure 1: Chromatogram of fatty acids on a 1 m x 1 mm i.d. column packed with pellicular graphitized carbon black (Winter 1964/1965). Mobile phase: ethanol-0.0001 M aqueous sodium hydroxide mixture.
A particular problem inherent in working with such small-diameter columns and with low mobile phase flow rates was extracolumn band broadening due to dead volumes, particularly in the detector. Csaba's work was helped greatly by an experimental, small-volume refractive index detector with a 10-μL flow cell that we provided to Csaba. This detector was developed around that time by Emmett Watson, probably the best development engineer we ever had at Perkin-Elmer, and we gave it to Csaba to help his development work. (Unfortunately, it has never been produced and marketed by the company.)
In the subsequent months, Csaba further improved his set-up, expanding it into a full-fledged scientific instrument. (Figure 2). The eluent (mobile phase) was contained in a reservoir in a thermostated air bath that could be heated or cooled. The columns were made of 1 mm i.d., 1/16 in. o.d. stainless steel tubes packed with the pellicular packing and coiled onto a thin-walled perforated cylinder. A short precolumn (one coil of the tube) was located before the injector, which consisted of a10-μL sliding valve and could be filled with a syringe. In the final system, Csaba used a Hitachi–Perkin-Elmer model 139 UV–vis spectrophotometer as the detector, with a 5-μL flow cell. This cell was made by Csaba and consisted of a Swagelock GC fitting with quartz windows. It was an engineering marvel. It might be interesting to note here that the first high-performance UV detector specially built for use in modern LC and described in 1968 by Kirkland (20) had a flow-through cell of 7.4-μL volume. The eluent was transported through the system by a Milton Roy Minipump. For gradient elution, a second pump also could added to the system.

Figure 2: The high performance liquid chromatograph developed by Csaba Horváth (Summer 1965).
Csaba finished the development of this instrument by the summer of 1965. Thus, this year we celebrate the 40-year anniversary of the first modern, high-pressure liquid chromatograph.
The preliminary investigations with the pellicular, graphitized carbon black packing were promising. However, Csaba had an unexpected problem with his environment. At that time, their laboratory was located in a hospital building and there were constant complaints due to pollution by the subsieve carbon dust formed when preparing the pellicular column packing. Therefore, he had to look for more "environment friendly" material. In the Medical School, ion-exchange resins already have been used in chromatographic separations and thus, he decided to use these as the stationary phase. First, he coated pellicular support particles with a liquid ion exchanger; however, such columns were not very stable. Therefore, Csaba decided instead to prepare pellicular particles coated with various porous solid ion-exchange resin layers, synthesized in situ. He also was looking for some biologically important problem to demonstrate the feasibility and usefulness of the new technique, and the analysis of nucleic acid derivatives was a logical choice. Their separation by classical ion-exchange chromatography had been shown first by Waldo Cohn at Oak Ridge National Laboratory, Oak Ridge, Tennessee, but the analysis time was very long, up to 100 h (21,22). Thus, while summarizing their work with the liquid ion exchangers in a short paper (23), Horváth and Lipsky started systematic investigations on the analysis of nucleic acids.
Parallel to their investigations on LC, Lipsky's group was also active in establishing a high-resolution MS–GC system that they planned to use in the investigations of the moon rocks. Because their system was novel in many respects, they planned to present a detailed report on it at the forthcoming Sixth International Symposium on Gas Chromatography, scheduled for September 1966, in Rome, Italy (24). This was the first time that the scope of the biennial symposium series was extended to include discussions on "associate techniques" (meaning primarily LC). This sounded like an excellent opportunity for Lipsky's group to present a second paper also, reporting on Csaba's results. However, although they finished the development of the instrument, work on nucleic acid analysis was still in progress, and being in an academic environment, they wanted to present a full report on their work. Therefore, it was felt that instead of contributing a formal paper, Csaba should present an interim report dealing with LC during the discussion section. I was present at the meeting and still remember the excitement. Without any doubt, he dominated the discussion and had to answer scores of questions raised by the standing room-only audience. The report on the discussion published in the proceedings of the symposium gave a brief summary of his presentation and emphasized that "there is no doubt that analysts will soon see a breakthrough in liquid chromatography" (25).
Next spring, Horváth and Lipsky, now together with a young colleague (Ben Preiss) who was well experienced in nucleic acid analysis, submitted a detailed report on their work to Analytical Chemistry (26). This fundamental paper consisted of three parts. First, a detailed theoretical discussion was given on the possibility of fast LC with both small-diameter packed and open-tubular columns, with the conclusion that the latter-type of columns were impractical in such systems. Based upon the van Deemter equation, this treatment included investigations on column characteristics and separation parameters, including temperature, and their influence on the analytical results. Next, the developed high-pressure liquid chromatograph and the way of preparation of the pellicular column packings were described. Finally, a detailed report was given on the successful separation of ribonucleosides in less than 1 h. As the conclusion the paper stated, it was "possible to achieve fast separation of complex mixtures by a liquid chromatographic technique similar in speed, resolution and quantitative range to gas chromatography." Without any doubt, this paper represents the start of modern LC.
The investigations of Horváth and Lipsky on high-pressure, fast LC and nucleic acid analysis did not end with this publication. In a paper submitted on October 20, 1968, to the Journal of Chromatographic Science and presented in January 1969 at the Fifth International Symposium on Advances in Chromatography in Las Vegas Nevada, they elaborated further on column design (27). This paper can serve as a model for how all aspects of column parameters should be explored. Further improvements in ribonucleoside analysis were reported in a parallel paper submitted on February 24, 1969, to Analytical Chemistry (28). In this paper, the separation of the four ribonucleosides in 5–6 min was demonstrated. Figure 3 shows two chromatograms from this paper. It might be interesting to point out that these analyses were carried out at elevated temperatures. Until recently, most LC analyses have been performed at room temperature. At the beginning, Csaba already showed the advantages of considering temperature as one of the variables.
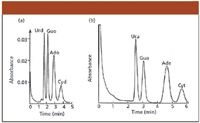
Figure 3: Fast analysis of (a) ribonucleotides and (b) nucleic acid bases. Column: 1 mm i.d. packed with pellicular cation exchange resin, sieve fraction #270/325; column length: (a) 151.7 cm, (b) 300.7 cm. Eluent: 0.02 M ammonium phosphate buffer (pH 5.5). Inlet pressure and flow rate: (a) 1925 psi, 35.5 mL/h, (b) 2380 psi, 33.4 mL/h; temperature: (a) 39 °C, (b) 68 °C; sample size: (a) 300 pmol, (b) 800 pmol of each component. UV detection at 254 nm; full scale response: (a) 0.04 AU, (b) 0.02 AU Peaks: Urd = uridine, Guo = guanosine, Ado = adenosine, Cyd = cytidine, Ura = urasil, Gua = guanine, Ada = adenine, Cyt = cytosine. From (28), reprinted with permission from Analytical Chemistry.
The Rapid Spreading of HPLC
Within a short time after the publication of the first paper by the Yale group, Picker Nuclear Company (White Plains, New York) built a commercial instrument based upon their work. Unfortunately, however, their interest was fairly narrow, and the company also felt that their customers would shy away from a "liquid chromatograph." Therefore, they restricted the instrument, calling it a Nucleic Acid Analyzer (model LCS 1000). However, other instrument companies soon started to pick up the development and by the end of the 1960s, several instruments were available commercially.
By 1969, pellicular, controlled-porosity support materials had been developed by Kirkland at DuPont (Wilmington, Delaware) and introduced commercially under the trade name Zipax (29,30). These support particles could be coated with a liquid stationary phase, just like the column packings used in GC. Coated support particles, however, created problems in LC because of the solubility of the stationary phases in the liquid mobile phase. Therefore, attempts were made to bond the stationary phase chemically to the support particle surface. In their paper (27), Horváth and Lipsky also pointed out this possibility. The breakthrough in this field was due to Kirkland's group, who developed such column packings with a variety of functional groups (31). The availability of stable column material with covalently bonded long alkyl chains also made the revival of reversed-phase LC possible. In this field, Csaba eventually achieved his fundamental contribution by laying down the theoretical basis of the technique, based upon the solvophobic theory. His 1976 paper on this subject (32), coauthored with Wayne Melander and Imre Molnár, is the most widely cited paper in our field (6). However, that is another story . . .
I cannot finish these recollections on this exciting time without a few words about Csaba's contributions to the vernacular of LC. In their original paper (26), they spoke about "fast liquid chromatography," and this characterization of the new technique also was emphasized in papers by other authors published soon afterward. By 1969, Horváth and Lipsky changed the name to "high-pressure liquid chromatography" (27). This term also was used by H. Felton of DuPont in his paper presented at the same symposium describing a new liquid chromatograph (33). The change to "high performance liquid chromatography" occurred in a paper presented by Csaba at the 1970 Pittsburgh Conference (34).
According to my best knowledge, the abbreviation "HPLC" was first used to characterize the new technique in the paper of Horváth and Lipsky, presented at the 1969 Las Vegas Symposium and published in its printed publication (27). It became widespread after Csaba's 1970 Pittsburgh Conference paper, in which he used this acronym consistently in his slides. Another term introduced in that paper was "isocratic" for constant-strength mobile phase. Within a short time, both terms became the universally used international expressions of modern HPLC.
In his recent paper discussing Csaba's contributions, Tyge Greibokk of the University of Oslo, Oslo, Norway, stated that "it would be difficult to find another person who had a greater impact on liquid chromatography than Csaba Horváth" (7). There is nothing to add to this statement. Today, when HPLC is the most universally used laboratory technique, every liquid chromatograph commemorates the scientist who developed the first modern liquid chromatograph and initiated the unparalleled evolution of the technique.
Acknowledgments
Figures 1 and 2 are from the author's collection. Figure 3 is from
Analytical Chemistry
, and Figure 4 was provided by Dr. Imre Molnár. The author would like to express his gratitude for the use of these figures.

Figure 4: Csaba Horváth with (left) Günther Bonn and (right) Imre Molnár in December 2002. Molnár (now at Molnár Institute, Berlin, Germany) was a postdoctoral associate in Professor Horváths laboratory from 1975 to 1977, at the time of the development of the solvophobic theory, while Bonn, Professor at the University of Innsbruck, Innsbruck, Austria, was a visiting scholar in the second part of the 1980s
References
(1) Cs. Horváth,
75 Years of Chromatography — A Historical Dialogue
, L.S. Ettre and A. Zlatkis, eds. (Elsevier, Amsterdam, The Netherlands, 1979), pp. 151–158.
(2) Cs. Horváth, Chromatography — A Century of Discovery 1900—2000, C.W. Gehrke, R.L. Wixom, and E. Bayer, eds. (Elsevier, Amsterdam, The Netherlands, 2001), pp. 237–248.
(3) I. Molnár, LCGC Eur.17, 418–421 (2004).
(4) K. Valko, Chromatographia59, 527–528 (2004).
(5) H. Kalász, J. Chromatogr. Sci.42, 340–341 (2004).
(6) G. Guiochon and L.A. Beaver, J. Chromatogr., A 1043, 123–126 (2004).
(7) T. Greibokk, J. Separ. Sci. 27, 1249–1254 (2004).
(8) F. Svec, J. Separ. Sci. 27, 1255–1272 (2004).
(9) Chem. Eng. News 39, 76 (July 3, 1961).
(10) A.J.P. Martin and R.L.M. Synge, Biochem. J. 35, 1358–1368 (1941).
(11 A.T. James and A.J.P. Martin, Biochem. J. 50, 679–690 (1952).
(12) R. Consden, A.H. Gordon, and A.J.P. Martin, Biochem. J. 38, 224–232 (1944).
(13) J.C.Giddings, Anal. Chem. 35, 2215–2216 (1963).
(14) S.R. Lipsky, 75 Years of Chromatography — A Historical Dialogue, L.S. Ettre and A. Zlatkis, eds. (Elsevier, Amsterdam, The Netherlands, 1979), pp. 265–276.
(15) M.J.E. Golay, Gas Chromatography 1960, R.P.W. Scott, ed. (Edinburgh Symposium Butterworths, London, U.K., 1960), pp. 139–143.
(16) I. Halász and Cs. Horváth, Anal. Chem. 35, 499–505 (1963).
(17) I. Halász and Cs. Horváth, Nature (London) 197, 71–72 (1963).
(18) I. Halász and Cs. Horváth, Anal. Chem. 36, 1178–1186m (1964).
(19) I. Halász and Cs. Horváth, Anal. Chem. 36, 2226–2229 (1964).
(20) J.J. Kirkland, Anal. Chem.40, 391–396 (1968).
(21) W.E. Cohn, Science 109, 377–378 (1949).
(22) W.E. Cohn, J. Am. Chem. Soc. 72, 1471–1478 (1950).
(23) Cs. Horváth and S.R. Lipsky, Nature (London) 322, 748–749 (1966).
(24) S.R. Lipsky, W.J.H. McMurray, and Cs. Horváth, Gas Chromatography 1966, A.B. Littlewood, ed. (Rome Symposium, Institute of Petroleum, London, U.K., 1967) pp. 299–317.
(25) J.V. Mortimer, Liquid Chromatography Discussion Section. Gas Chromatography 1966, A.B. Littlewood, ed. (Rome Symposium, Institute of Petroleum, London, U.K., 1967) pp. 414–418.
(26) Cs. Horváth, B.A. Preiss, and S.R. Lipsky, Anal. Chem. 39, 1422–1428 (1967).
(27) Cs. Horváth and S.R. Lipsky, J. Chromatogr. Sci. 7, 109-116 (1969).
(28) Cs. Horváth and S.R. Lipsky, Anal. Chem. 41, 1227–1234 (1969).
(29) J.J. Kirkland, Anal. Chem. 41, 218–220 (1969).
(30) J.J. Kirkland, J. Chromatogr. Sci. 7, 7–12 (1969).
(31) J.J. Kirkland and J.J. DiStefano, J. Chromatogr. Sci. 8, 309–314 (1970).
(32) Cs. Horváth, W. Melander, and I. Molnár, J. Chromatogr. 215, 129–156 (1976).
(33) H. Felton, J. Chromatogr. Sci.7, 13–16 (1969).
(34) Cs. Horváth, 21st Pittsburgh Conference, Cleveland, Ohio, March 2–6, 1970.
Leslie S. Ettre From 1988 to 2004, "Milestones in Chromatography" editor Leslie S. Ettre was associated with the Chemical Engineering Department of Yale University (New Haven, Connecticut), first as an adjunct professor and then as a research fellow. Previously, he had been with the Perkin-Elmer Corporation for 30 years. He is currently a member of LCGC's editorial advisory board.

Newsletter
Join the global community of analytical scientists who trust LCGC for insights on the latest techniques, trends, and expert solutions in chromatography.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)